Incomplete resection after macroscopic radical endoscopic resection of T1 colorectal cancer—should a paradigm-changing approach to address the risk be considered?
Compared with laparoscopic resection, endoscopic resection was found to be cost-effective in the management of complex colon polyps. The effectiveness was due to superior technical success and reduced adverse event rates associated of endoscopic resection, and to the higher cost of laparoscopic resection (1). The economic analysis could explain why endoscopic interventions such as endoscopic mucosal resection and endoscopic submucosal dissection have gained popularity and are being increasingly incorporated into the management of T1 colorectal cancer (CRC). T1 CRC is one that has grown through the muscularis mucosa and extends into the submucosa (2).
In the 2010 guidelines for the treatment of CRC from the Japanese Society for Cancer of the Colon and Rectum (JSCCR), the criteria for identifying curable T1 CRC after endoscopic resection included well/moderately differentiated or papillary histologic grade, no vascular invasion, submucosal invasion depth <1,000 µm and budding grade 1 (low grade). In one report aimed to expand these criteria, 499 T1 CRC, resected endoscopically or surgically, were analyzed. Lymph node metastasis was found in 41 (8.2%). The incidence of lymph node metastasis was significantly higher in lesions with poorly differentiated/mucinous adenocarcinoma, submucosal invasion ≥1,800 µm, vascular invasion, and high-grade tumor budding. Multivariate logistic regression analysis revealed these variables to be independent risk factors for lymph node metastasis. When cases that met three of the JSCCR 2010 criteria (i.e., all but invasion <1,000 µm) were considered together, the incidence of lymph node metastasis was only 1.2% (3/249, 95% CI: 0.25–3.48%), and there were no cases of lymph node metastasis without submucosal invasion to a depth of ≥1,800 µm. The investigators concluded that even in cases of CRC with deep submucosal invasion, the risk of lymph node metastasis is minimal under certain conditions. Thus, even for such cases, endoscopic incisional biopsy could be suitable if complete en bloc resection was achieved (3).
The risk of lymph node metastasis appears to be dependent on the characteristics of the T1 CRC. In one study 435 patients with T1 CRC were treated by surgical or endoscopic resection. In the surgically resected group (n=324), lymph node metastasis was detected in 42 patients (13.0%). Grade 3, angiolymphatic invasion, budding, and the absence of background adenoma were factors associated with lymph node metastasis in univariate and multivariate analyses (P<0.05). In the endoscopically resected group (n=111), three of 50 patients with high risk were diagnosed with lymph node metastasis during the follow-up period. There was no lymph node metastasis in the endoscopically resected group with low risk (4). Thus, lymph node metastasis is dependent on the T1 CRC and not on the mode (endoscopic or surgical) of treatment. On the basis of another retrospective study of patients who underwent endoscopic resection for T1 CRC, those with tumors with only submucosal invasion were at low risk for cancer recurrence. However, patients with high-risk tumor features have greater risks for cancer recurrence and benefit from subsequent surgery (5).
A recent Japanese report examined T1 CRC patients treated during 1992–2008 and who had ≥5 years of follow-up (6). Patients who did not meet the curative criteria after endoscopic resection according to the JSCCR guidelines were defined as “non-endoscopically curable” and classified into three groups: endoscopic resection alone, additional surgery after endoscopic resection, and surgical resection alone. Table 1 summarizes the findings. Age ≥65 years, protruded gross type, positive lymphatic invasion, and high budding grade were significant predictors of recurrence in these patients. The authors maintained that the findings supported the JSCCR criteria for endoscopically curable T1 CRC. Endoscopic resection for T1 CRC did not worsen the clinical outcomes of patients who required additional surgical resection.
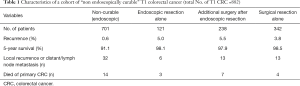
Full table
To address the controversy over the optimal management for T1 CRC, another study (7) compared initial endoscopic resection with or without additional surgery, or initial surgery for T1 CRC, and assessed risk factors for lymph node metastases and long-term recurrence. This was a registration study of patients diagnosed with T1 CRC from 1995–2011 in the southeast area of The Netherlands (n=1,315). High-risk histology was defined as the presence of poor differentiation, lymphangio-invasion, and/or deep submucosal invasion. Findings are shown in Table 2. Endoscopic resection was performed in 590 patients (44.9%); of these, 220 (16.7%) underwent additional surgery. Initial surgery was performed in 725 patients (55.1%). The risk of lymph node metastases was higher in T1 CRC with histologic risk factors (15.5% vs. 7.1% without histologic risk factors; odds ratio, 2.21; 95% CI: 1.33–3.70). The only independent risk factor for long-term recurrence was a positive resection margin (hazard ratio, 6.88; 95% CI: 2.27–20.87). Based on the population analysis, the investigators concluded that additional surgery after endoscopic resection should be considered only for patients with high-risk histology or a positive resection margin.
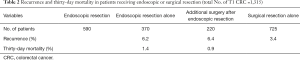
Full table
Patients with T1 CRC had a distinctly higher incidence of local recurrence after endoscopic resection or local resection. Explicit workup in terms of risk classification is crucial to reducing the risk of local and systemic recurrence. A non-radical approach should be only a second option for patients with T1 CRC, namely, those solely in clearly low-risk situations or those with distinct co-morbidities (8). Local resection may be effective and oncologically safe in low-risk T1 CRC. Although additional surgery should be recommended for the locally resected high-risk T1 CRC cases, intensive surveillance without additional surgery and timely salvage operation may offer another treatment option, if vascular invasion is negative (9). Data in another report did not support an increased risk of lymph node metastasis or recurrence after secondary surgery compared with primary surgery. Therefore, an attempt for an en bloc resection of a possible T1 CRC without evident signs of deep invasion seems justified in order to prevent surgery of low-risk T1 CRC in a significant proportion of patients (10).
With this backdrop, a recent retrospective report from the Dutch T1 CRC Working Group published in the American Journal of Gastroenterology continued to describe promising results of macroscopic radical endoscopic resection of T1 CRC (11). Data from patients treated between 2000 and 2014 with macroscopic complete endoscopic resection of T1 CRC were collected from 13 hospitals in the Netherlands. Incomplete resection was defined as local recurrence at the polypectomy site during follow-up, or malignant tissue in the surgically resected specimen when secondary surgery was performed. A total of 877 patients with a median follow-up time of 36.5 months (interquartile range, 16.0–68.3) were included, in whom secondary surgery was performed in 358 patients (40.8%). Incomplete resection was observed in 30 patients (3.4%; 95% CI: 2.3–4.6%). Incomplete resection rate was 0.7% (95% CI: 0–2.1%) in low-risk T1 CRC vs. 4.4% (95% CI: 2.7–6.5%) in high-risk T1 CRC (P=0.04). Overall adverse outcome rate (incomplete resection or metastasis) was 2.1% (95% CI: 0–5.0%) in low-risk T1 CRC vs. 11.7% (95% CI: 8.8–14.6%) in high-risk T1 CRC (P=0.001). Piecemeal resection (adjusted odds ratio, 2.60; 95% CI: 1.20–5.61, P=0.02) and non-pedunculated morphology (adjusted odds ratio 2.18; 95% CI: 1.01–4.70, P=0.05) were independent risk factors for incomplete resection. Among patients in whom no additional surgery was performed, 41.7% (95% CI: 20.8–62.5%) died as a result of recurrent cancer. The authors concluded that in the absence of histological high-risk factors, a ‘wait-and-see’ policy with limited follow-up is justified. Piecemeal resection and non-pedunculated morphology are independent risk factors for incomplete endoscopic resection of T1 CRC.
An editorial in Translational Gastroenterology and Hepatology commented extensively on an earlier phase of this work (10). The editorial concluded evolving innovative methods and new devices may change traditional paradigms to allow minimally invasive intervention for CRC in the future (12).
Underwater resection is one such paradigm-changing approach. Traditional endoscopic mucosal resection or endoscopic submucosal dissection are performed in a gas (air or carbon dioxide) filled colonic lumen. Underwater resection has evolved from two different modes of water use during colonoscopy. In one mode, water exchange was used by investigators to minimize insertion pain. A difficult to capture pedunculated polyp due to spasms in the sigmoid colon was encountered. Water infusion distention provided sufficient space around the polyp for its successful capture by snare, polypectomy and retrieval (13). The underwater resection approach has been extended safely to other pedunculated and non-pedunculated polyps (14) and for salvage resection (15). The ongoing practice of underwater polypectomy by colonoscopists in multiple countries confirm the feasibility and acceptability of the novel approach (16). In another mode, investigators filled the colonic lumen with water for endoscopic ultrasound assessment of colonic lesions. The following salient observations were made. The ultrasound images have shown that the colonic wall retains its native thickness of 3–4 mm, the muscularis propria retains a circular configuration and does not follow the involutions of the mucosa and submucosa. This configuration is maintained even during peristaltic contractions. On both ultrasound and endoscopic viewing the mucosa and submucosa appear to “float” away from the deeper muscularis propria. This is mainly an effect of the gravity-free environment of water (Binmoeller). In one report of underwater endoscopic mucosal resection without submucosal injection, 60 patients with 62 large sessile colorectal polyps were described. The mean/median polyp size was 34/30 mm, and the mean/median resection time was 21/18 minutes. Histology revealed tubular adenoma (n=22), tubulovillous adenoma (n=19), villous adenoma (n=4), serrated adenoma (n=11), and high-grade dysplasia/carcinoma in situ (n=6). The mean/median interval until a follow-up colonoscopy in 54 patients (90%) was 20.4/15.2 weeks. One of 54 patients (2%) had an adenoma smaller than 5 mm outside of the post-resection scar, consistent with a residual lesion missed on index underwater endoscopic mucosal resection. The technique was safe, and the early recurrence rate appeared low (17). Others described underwater endoscopic mucosal resection as easy to implement (18,19), and the third way for en bloc resection of colonic lesions (20). To date, only two cases of perforation following underwater endoscopic mucosal resection have been reported (21,22).
In a more recent report, 289 colorectal polyps were removed by a single endoscopist from 7/2007 to 2/2015 using endoscopic mucosal resection (in air-fill lumen) or underwater endoscopic mucosal resection. In total, 135 polyps (endoscopic mucosal resection: 62, underwater endoscopic mucosal resection: 73) that measured ≥15 mm and had not undergone prior attempted polypectomy were evaluated for rates of complete macroscopic resection and adverse events. And, 101 of these polyps (endoscopic mucosal resection: 46, underwater endoscopic mucosal resection: 55) had at least one follow-up colonoscopy and were studied for rates of recurrence and the number of procedures required to achieve curative resection. The rate of complete macroscopic resection was higher following underwater endoscopic mucosal resection compared to endoscopic mucosal resection (98.6% vs. 87.1%, P=0.012). Underwater endoscopic mucosal resection had a lower recurrence rate at the first follow-up colonoscopy compared to endoscopic mucosal resection (7.3% vs. 28.3%, OR 5.0 for post-endoscopic mucosal resection recurrence, 95% CI: 1.5–16.5, P=0.008). Underwater endoscopic mucosal resection required fewer procedures to reach curative resection than endoscopic mucosal resection (mean of 1.0 vs. 1.3, P=0.002). There was no significant difference in rates of adverse events. Underwater endoscopic mucosal resection appears superior to endoscopic mucosal resection for the removal of large colorectal polyps in terms of rates of complete macroscopic resection and recurrent (or residual) abnormal tissue. Compared to conventional endoscopic mucosal resection, underwater endoscopic mucosal resection may offer increased procedural effectiveness without compromising safety in the removal of large colorectal polyps without prior attempted resection (23).
The impact of underwater resection on completeness of resection and recurrence of T1 CRC is not known. Studies of underwater resection employing complete resection rate as the primary outcome and recurrence rate as secondary outcome deserve to be performed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Law R, Das A, Gregory D, et al. Endoscopic resection is cost-effective compared with laparoscopic resection in the management of complex colon polyps: an economic analysis. Gastrointest Endosc 2016;83:1248-57. [Crossref] [PubMed]
- Colorectal Cancer Stages. American Cancer Society. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/staged.html
- Nakadoi K, Tanaka S, Kanao H, et al. Management of T1 colorectal carcinoma with special reference to criteria for curative endoscopic resection. J Gastroenterol Hepatol 2012;27:1057-62. [Crossref] [PubMed]
- Suh JH, Han KS, Kim BC, et al. Predictors for lymph node metastasis in T1 colorectal cancer. Endoscopy 2012;44:590-5. [Crossref] [PubMed]
- Yoshii S, Nojima M, Nosho K, et al. Factors associated with risk for colorectal cancer recurrence after endoscopic resection of T1 tumors. Clin Gastroenterol Hepatol 2014;12:292-302.e3. [Crossref] [PubMed]
- Tamaru Y, Oka S, Tanaka S, et al. Long-term outcomes after treatment for T1 colorectal carcinoma: a multicenter retrospective cohort study of Hiroshima GI Endoscopy Research Group. J Gastroenterol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Belderbos TD, van Erning FN, de Hingh IH, et al. Long-term recurrence-free survival after standard endoscopic resection versus surgical resection of submucosal invasive colorectal cancer: a population-based study. Clin Gastroenterol Hepatol 2017;15:403-11.e1. [Crossref] [PubMed]
- Kogler P, Kafka-Ritsch R, Öfner D, et al. Is limited surgery justified in the treatment of T1 colorectal cancer? Surg Endosc 2013;27:817-25. [Crossref] [PubMed]
- Nam MJ, Han KS, Kim BC, et al. Long-term outcomes of locally or radically resected T1 colorectal cancer. Colorectal Dis 2016;18:852-60. [Crossref] [PubMed]
- Overwater A, Kessels K, Elias SG, et al. Endoscopic resection of high-risk T1 colorectal carcinoma prior to surgical resection has no adverse effect on long-term outcomes. Gut 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Backes Y, de Vos Tot Nederveen Cappel WH, van Bergeijk J, et al. Risk for incomplete resection after macroscopic radical endoscopic resection of T1 colorectal cancer: a multicenter cohort study. Am J Gastroenterol 2017;112:785-96. [Crossref] [PubMed]
- Fujihara S, Mori H, Kobara H, et al. Endoscopic treatment for high-risk T1 colorectal cancer: is it better to begin with endoscopic or surgical treatment? Transl Gastroenterol Hepatol 2017;2:39. [Crossref] [PubMed]
- Anderson JM, Goel GA, Cohen H, et al. Water infusion distention during colonoscopy is a safe alternative technique to facilitate polypectomy in a "difficult location". J Interv Gastroenterol 2013;3:137-40.
- Ocampo LH, Kunkel DC, Yen A, et al. Underwater hot and cold snare polypectomy can be safely executed during water exchange colonoscopy. J Interv Gastroenterol 2013;3:104-6. [Crossref]
- Friedland S, Leung FW. Underwater endoscopic mucosal resection as a salvage treatment after unsuccessful standard endoscopic mucosal resection in the colon. J Interv Gastroenterol 2013;3:93-5. [Crossref]
- Leung FW, Amato A, Cadoni S, et al. Novel developments in water-aided techniques for education and research (WATER) in colonoscopy. Am J Gastroenterol 2015;110:S556.
- Binmoeller KF, Weilert F, Shah J, et al. "Underwater" EMR without submucosal injection for large sessile colorectal polyps (with video). Gastrointest Endosc 2012;75:1086-91. [Crossref] [PubMed]
- Wang AY, Flynn MM, Patrie JT, et al. Underwater endoscopic mucosal resection of colorectal neoplasia is easily learned, efficacious, and safe. Surg Endosc 2014;28:1348-54. [Crossref] [PubMed]
- Curcio G, Granata A, Ligresti D, et al. Underwater colorectal EMR: remodeling endoscopic mucosal resection. Gastrointest Endosc 2015;81:1238-42. [Crossref] [PubMed]
- Amato A, Radaelli F, Spinzi G. Underwater endoscopic mucosal resection: The third way for en bloc resection of colonic lesions? United European Gastroenterol J 2016;4:595-8. [Crossref] [PubMed]
- Hsieh YH, Koo M, Leung FW. A patient-blinded randomized, controlled trial (RCT) comparing air insufflation (AI), water immersion (WI) and water exchange (WE) during minimally sedated colonoscopy. Am J Gastroenterol 2014;109:1390-400. [Crossref] [PubMed]
- Ponugoti PL, Rex DK. Perforation during underwater EMR. Gastrointest Endosc 2016;84:543-4. [Crossref] [PubMed]
- Schenck RJ, Jahann DA, Patrie JT, et al. Underwater endoscopic mucosal resection is associated with fewer recurrences and earlier curative resections compared to conventional endoscopic mucosal resection for large colorectal polyps. Surg Endosc 2017. [Epub ahead of print]. [Crossref] [PubMed]
Cite this article as: Leung FW. Incomplete resection after macroscopic radical endoscopic resection of T1 colorectal cancer—should a paradigm-changing approach to address the risk be considered? Transl Gastroenterol Hepatol 2017;2:69.


