Hepatocellular carcinoma: when is liver transplantation oncologically futile?
Introduction
Liver transplantation (LT) in patients with “early stage” hepatocellular carcinoma (HCC) with a single tumour ≤5 cm in diameter or a maximum of three tumours ≤3 cm without evidence of vascular invasion (so called Milan criteria) is associated with excellent long-term survival rates reaching over 70% after 5 years (1,2). The implementation of these selection criteria resulted in the general acceptance of LT as a standard treatment in patients with early HCC but excluded a significant number of patients with more advanced tumors from a potentially curative treatment. Although tumor stage within the Milan criteria remains a prerequisite for prioritizing patients on the waiting list in United Network for Organ Sharing (UNOS) and Eurotransplant regions, a large number of patients with tumors beyond the Milan criteria underwent LT. In fact, a retrospective study including 1,556 HCC patients in 36 American, European and one Asian center showed that 71% of patients underwent LT despite a tumor stage exceeding the Milan criteria (3). To balance the possible benefit of LT for HCC patients exceeding the Milan criteria against the risk of HCC recurrence and unfavourable post-transplant survival, two different strategies have been evaluated: expansion of transplant criteria and downstaging. Several studies have demonstrated that both strategies can achieve low tumor recurrence and 5-year survival rates of over 70% despite (initial) tumor stages exceeding the Milan criteria (3-13).
The main focus of this article is to review LT for HCC in the light of recurrence rates and to explore at what tumor stage LT becomes futile. The introduction of the Milan Criteria more than 20 years ago and the subsequent progressive expansion of selection criteria marks this critical appraisal of utility and futility of LT for HCC. Although tumor number and size, basis of the widely accepted Milan criteria, correlate with tumor grading and microvascular invasion, other factors have been identified as equally or more predictive for tumor recurrence (14). This is especially true as pre-transplant tumor staging underestimates tumor burden in up to 23% of patients and its accuracy depends on the modality of imaging (15). Computed tomography (CT) can miss up to one-fifth of HCC lesions that are detected by subsequent magnetic resonance imaging (16). The following review discusses broadly available tumor characteristics that are better indicators of tumor biology and oncologic futility than just number and size.
Futility rule #1: extrahepatic metastases and macrovascular invasion
Extrahepatic tumor spread cannot be cured by LT and represents a clear contraindication to LT. Also macrovascular invasion is considered an absolute contraindication to LT as it is an independent risk factor for HCC recurrence and associated with decreased survival (17,18). Hence the Milan but also extended criteria like the University of California San Francisco (UCSF) or the up-to-seven criteria exclude patients with macrovascular invasion (1,3,9). Nevertheless, this dogma has been questioned recently. In our own series, 17 patients underwent LT with macrovascular invasion in pre-LT imaging of whom 10 survived without HCC recurrence (18). The 5-year overall survival was 56%. Although absolute numbers were small, none of the patients with complete response to neoadjuvant treatment had HCC recurrence. This “oncologically favourable” outcome has been recently confirmed by several case series (19-22). Of a total of 21 patients who underwent LT with macrovascular invasion, 8 patients developed HCC recurrence. Overall survival after 5 years was 64% in the largest series of patients with angioinvasive HCC (20). Main portal vein invasion was identified as the single most important risk factor for HCC recurrence, whereas a score including alpha-fetoprotein (AFP) and protein induced by vitamin K absence/antagonist-II (PIVKA-II) was predictive for recurrence free survival. Although evidence of macrovascular invasion should still be considered a (relative) contraindication to LT, in selected patients without any additional risk factors LT may improve survival compared to palliative treatment. Therefore LT could be considered especially if a living donor is available or local resources allow the use of donor organs also for patients with a survival chance below that normally assumed for high quality transplant programs.
Futility rule #2: progressive disease despite (locoregional) therapy [at least in tumor stages beyond Milan/Barcelona Clinic Liver-Cancer (BCLC) A] and too short waiting periods
Prospective trials have shown that selected patients with a tumor burden exceeding the Milan and even the UCSF criteria can undergo LT with a recurrence risk and a 5-year survival comparable to patients initially presenting within the Milan criteria if they are successfully downstaged (7,10). In these trials, maximum tumor size for inclusion in downstaging protocols was limited and a recurrence free period of at least 3 months was mandatory before LT listing. In contrast, LT despite failure to downstaging is associated with an increased risk of recurrence. Data from patients with BCLC stage B and C show that progressive disease despite locoregional therapy is associated with an about 5-fold increase in recurrence rate (18). When also BCLC 0 and BCLC A patients were included in the analysis, 5-year overall survival was 62% in patients with progressive disease compared to 87% in patients with complete remission. In another recent study in patients transplanted beyond the Milan criteria, the recurrence risk was 4.9-fold higher in those patients with poor response to locoregional treatment when compared with successfully downstaged patients (23). The importance of response to locoregional treatment was further demonstrated by the fact that patients within the Milan criteria but progressive disease according to mRECIST had a worse outcome comparable to those of the patients with HCC beyond Milan criteria at both HCC diagnosis and transplantation. The finding that tumor progression on the waiting list is a strong predictor of HCC recurrence even when tumor stage is within the Milan criteria was confirmed in independent cohorts (24,25). Response to locoregional treatment can therefore be considered a surrogate of tumor biology, because patients without response are more likely to exhibit poorly differentiated tumors and microvascular invasion—both important predictors of recurrence (see futility rule #3) (18,23).
To identify patients with aggressive and thus unfavourable tumor biology, the time interval between treatment and recurrence is crucial. Recurrence within the first 8–12 months after liver resection is an independent risk factor for recurrence after subsequent salvage transplantation (26,27). In contrast, longer recurrence free intervals after pre-LT treatment select patients with a low post-LT recurrence risk. In a large analysis of >5,000 HCC patients in the UNOS region those who remained on the waiting list without recurrence for >120 days had a 40% reduced risk of post-LT recurrence compared to those with a shorter waiting time (28). This “sweet spot” of 6–18 months waiting time has also been demonstrated by a very recent study (29). Another analysis of UNOS data showed that a higher MELD score at LT was independently associated with lower post-LT mortality in HCC patients but with a higher mortality in non-HCC patients (30). In HCC patients, higher (exceptional) MELD scores indicate a longer waiting time whereas in non-HCC patients, higher MELD scores indicate more severely ill patients. Finally, post-LT outcome has been shown to be better in regions with long waiting times (median 7.6 months) compared to regions with short waiting times (median 1.6 months) despite patients in long waiting time regions were more likely to have larger tumors at listing and to receive expanded-criteria grafts (31). All these data suggest that expediting patients with HCC to transplant at a too fast rate may prevent the identification of rapidly progressing tumors with aggressive biology and therefore adversely affect patient outcomes. Hence the latest revision of the UNOS policy has introduced a mandatory 6-month waiting period prior to the application of MELD exception points to facilitate selection of patients with good tumor biology and to lower the risk of post-transplant recurrence (32).
Futility rule #3: presence of microvascular invasion or poor histologic differentiation
Tumor biology is the most important predictor of HCC recurrence risk where aggressive biology is indicated by poor histologic differentiation and microvascular invasion. Both are long known as strong predictors of HCC recurrence. Microvascular invasion doubled the hazard of death with a post-LT 5-year overall survival rate of 64% and 33% depending on the absence or presence of microvascular invasion in patients outside the up-to seven criteria (3). Microvascular invasion and tumor differentiation independently predicted HCC recurrence and their absence or presence stratified patients beyond the up-to-seven criteria in subgroups with lower (recurrence rate =24%) and higher (recurrence rate =45%) risk (33). Numerous more recent studies have confirmed that poor differentiation and microvascular invasion are associated with recurrence in patients within and beyond various transplant criteria (18,34-38). Conversely, the absence of these risk factors may justify LT also in patients with large HCC as shown by the Toronto experience. The Extended Toronto Criteria allow LT for patients with any number and any size of HCC lesions provided no evidence exists for vascular invasion or extrahepatic disease, no cancer-related constitutional symptoms are observed, and a targeted biopsy of the largest lesion does not show poor differentiation (39). Two studies evaluated these criteria in patients exceeding the Milan criteria and in patients exceeding the UCSF criteria and found 5-year overall survival rates of 69% and 66%, respectively, which was not significantly lower than in patients within the Milan criteria (39,40).
Futility rule #4: (persistently) increased AFP
Histology to assess tumor grading and microvascular invasion is not always available. And even if a biopsy is performed, microvascular invasion can be missed and tumor grading may not be uniformly across all lesions in patients with multilocular HCC. Therefore AFP is a well-established surrogate of tumor biology as it correlates with histologic grading and vascular invasion (14,41-43). Not surprisingly, a large number of studies have highlighted the importance of increased AFP values for the recurrence risk after LT (3,23,25-27,39,41-48). It is important to note that increased AFP values are not only a risk factor in patients beyond but also in patients within the Milan criteria. A study including >200 HCC patients within the Milan criteria showed a 5-year recurrence-free survival of 53% for patients with an AFP >1,000 ng/mL and of 80% for patients with an AFP ≤1,000 ng/mL (43). Applying an AFP level >1,000 ng/mL as a cut-off would have resulted in the exclusion of only 4.7% of the patients from LT but a 20% reduction in HCC recurrence. Furthermore, AFP should not be seen as a static variable. Especially when locoregional therapies are applied, AFP dynamics can stratify different risk populations. In a large cohort of nearly 7,000 patients with HCC listed for LT, patients with AFP levels >400 ng/mL at the time of listing who were downstaged to AFP ≤400 ng/mL had better intent-to-treat survival than patients failing to reduce and comparable survival to patients with AFP continuously ≤400 ng/mL (48). Similar results have been reproduced in a smaller, more recent study where a cut-off of 100 ng/mL was applied in a cohort that included also patients exceeding the UCSF and the up-to-seven criteria (41). Patients with an AFP persistently <100 ng/mL and those with initially high AFP dropping to <100 ng/mL before LT had a 5-year recurrence-free survival of 97–100%, whereas those with rising or persistently increased AFP had survival rates of 75% and 38%, respectively. This stratification was seen both in patients within and beyond the Milan criteria.
Although these studies confirm the role of AFP in risk stratification, they also illustrate the main problem when considering AFP as a possible exclusion criterion for LT: there is no consensual cut-off that can be universally applied to all cohorts. Even more, cut-offs may vary according to tumor stage. In an improved prognostic model including AFP, tumor number and size two different cut-offs distinguished recurrence risk according to the Milan status (42). In patients within the Milan criteria, an AFP >1,000 ng/mL identified patients with a 5-year risk of recurrence of 37%. For patients exceeding Milan criteria, the cut-off was >100 ng/mL and associated with a 5-year recurrence risk of 48%. Patients with an AFP below the respective cut-off had a low recurrence risk <15% in both subgroups.
Taken together, a markedly increased AFP concentration should be considered a contraindication to LT. This should not only apply to patients undergoing downstaging protocols or to patients included in extended criteria, but also to patients within the Milan criteria. The challenge remains to define an exact cut-off that excludes patients from LT.
Futility rule #5: positive positron emission tomography (PET)
Similar to AFP, functional imaging with 18F-FDG PET/CT is a non-invasive surrogate of tumor biology. Increased 18F-FDG uptake on PET is correlated with higher AFP concentrations, larger tumor size, poor differentiation and microvascular invasion (49-52). In a study correlating results from histological evaluation and PET imaging, a high level of 18F-FDG accumulation showed a sensitivity of 84.1% and a specificity of 75.0% for distinguishing between well- and poorly differentiated HCC (53). Hence it is not surprising that PET imaging can predict the risk of HCC recurrence after LT. Several studies found an increased recurrence risk in patients with PET positive HCC lesions (46,50,51). Two of these studies demonstrated that patients exceeding the Milan criteria but were PET negative had a comparable 5-year overall and recurrence free survival to patients within the Milan criteria (50,51). The predictive power of pre-LT PET examination could be increased by combination with serum AFP concentrations in a recent study including 123 patients within and beyond the Milan criteria (46). For PET imaging the cut-off was a ratio of 1:1.1 between maximum uptake of normal liver and maximum uptake of tumor and for AFP the cut-off of was set at 200 ng/mL. Three risk groups were defined: low risk with negative PET and negative AFP, intermediate risk with either PET or AFP positive and high risk with both positive. The high risk group had a dismal 5-year recurrence free survival both in Milan in and Milan out patients with 17% and 0%, respectively. The superiority of tumor biology (PET and AFP) compared to tumor morphology (Milan criteria) in prediction of recurrence risk was depicted by the hazard ratios. Whereas the hazard yielded by the use of Milan criteria was 1.35, the hazard ratio of the biologically high risk group compared to the low risk group was 29.06. The data from these studies demonstrate that 18F-FDG is a valuable tool in patient selection for LT. Whereas PET negativity may encourage LT in patients beyond classic selection criteria, PET positivity should be regarded as a contraindication especially when accompanied by further risk factors like positive AFP.
Futility rule #6: do not transplant when multiple risk factors are present
As indicated by the above mentioned study combining PET results and AFP levels, the combination of additional risk factors is associated with a sharp increase of recurrence rates and dismal prognosis after LT. The impact of additive risk factors has been nicely demonstrated by two recent studies. In the first study that included patients undergoing salvage LT after resection, microvascular invasion at the time of liver resection, a time interval to post-resection HCC recurrence of ≤12 months, an AFP level at LT >200 ng/mL, and having undergone LT outside of the UCSF criteria were independent risk factors for HCC recurrence after LT. Patients with no more than one risk factor had a 5-year recurrence-free survival rate of 71% compared to 16% in patients with two or more risk factors (27). The results of the second study, which only included patients within the Milan criteria undergoing liver resection or living-donor LT, were even more striking. Risk factors accounted for included tumor differentiation, microvascular invasion, AFP >200 ng/mL and a tumor size >3 cm. Patients without or with only one risk factor had a significantly lower HCC recurrence rate after LT compared to patients undergoing resection at similar 3-year overall survival. In patients with two or more risk factors, recurrence rates after LT were not better than after resection and reached up to 75% at 3 years. Notably, overall survival after LT was significantly worse compared to resection in these high risk patients (54). Given the large amount of available evidence, LT may be futile both from a transplant and an oncological point of view when unfavorable biological characteristics are present even in patients that would be eligible for LT when only morphological criteria were applied. Figure 1 shows a proposed algorithm for risk stratification based on tumor morphology and biology.
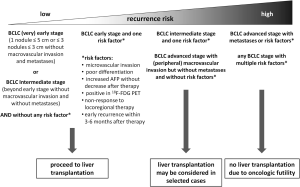
Conclusions
Tumor biology is the most important determinant of post-transplant tumor recurrence and survival. Selection criteria mainly based on tumor size and number insufficiently reflect tumor biology and are not ideally suited to define oncologic futility in the setting of LT for HCC. Vascular invasion on pre-LT imaging, progression despite locoregional therapy, increased AFP and positivity in 18F-FDG PET as well as the two histologic hallmarks of aggressive tumor behaviour, poor differentiation and microscopic vascular invasion, may be the better surrogates of an unfavourable tumor biology and a dismal outcome after LT. Especially the combination of risk factors poses a high recurrence risk and can render LT oncologically futile even in patients who would be eligible for transplantation according to morphology-based criteria like the Milan, UCSF or up-to-seven criteria. In contrast, patients exceeding these criteria can undergo LT without increased risk of recurrence if no other risk factors are present.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors haves no conflicts of interest to declare.
References
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [PubMed]
- Mazzaferro V, Bhoori S, Sposito C, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl 2011;17 Suppl 2:S44-57. [PubMed]
- Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35-43. [PubMed]
- Kaido T, Takada Y, Uemoto S.. Usefulness of the Kyoto Criteria as Selection Criteria for Living Donor Liver Transplantation for Hepatocellular Carcinoma. Liver Transpl 2010;16:538-40. [PubMed]
- Lei J, Wang W, Yan L. Downstaging advanced hepatocellular carcinoma to the Milan criteria may provide a comparable outcome to conventional Milan criteria. J Gastrointest Surg 2013;17:1440-6. [PubMed]
- Otto G, Herber S, Heise M, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl 2006;12:1260-7. [PubMed]
- Ravaioli M, Grazi GL, Piscaglia F, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant 2008;8:2547-57. [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl 2002;8:765-74. [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [PubMed]
- Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology 2015;61:1968-77. [PubMed]
- Xu X, Lu D, Ling Q, et al. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Gut 2016;65:1035-41. [PubMed]
- Duffy JP, Vardanian A, Benjamin E, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg 2007;246:502-9; discussion 9-11. [PubMed]
- Chapman WC, Majella Doyle MB, Stuart JE, et al. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg 2008;248:617-25. [PubMed]
- Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl 2005;11:1086-92. [PubMed]
- Costentin CE, Amaddeo G, Decaens T, et al. Prediction of hepatocellular carcinoma recurrence after liver transplantation: Comparison of four explant-based prognostic models. Liver Int 2017;37:717-26. [PubMed]
- Rostambeigi N, Taylor AJ, Golzarian J, et al. Effect of MRI Versus MDCT on Milan Criteria Scores and Liver Transplantation Eligibility. AJR Am J Roentgenol 2016;206:726-33. [PubMed]
- Andreou A, Bahra M, Schmelzle M, et al. Predictive factors for extrahepatic recurrence of hepatocellular carcinoma following liver transplantation. Clin Transplant 2016;30:819-27. [PubMed]
- Finkenstedt A, Vikoler A, Portenkirchner M, et al. Excellent post-transplant survival in patients with intermediate stage hepatocellular carcinoma responding to neoadjuvant therapy. Liver Int 2016;36:688-95. [PubMed]
- Choi JY, Yu JI, Park HC, et al. The possibility of radiotherapy as downstaging to living donor liver transplantation for hepatocellular carcinoma with portal vein tumor thrombus. Liver Transpl 2017;23:545-51. [PubMed]
- Lee KW, Suh SW, Choi Y, et al. Macrovascular invasion is not an absolute contraindication for living donor liver transplantation. Liver Transpl 2017;23:19-27. [PubMed]
- Levi Sandri GB, Ettorre GM, Colasanti M, et al. Hepatocellular carcinoma with macrovascular invasion treated with yttrium-90 radioembolization prior to transplantation. Hepatobiliary Surg Nutr 2017;6:44-8. [PubMed]
- Borentain P, Gregoire E, Louis G, et al. Successful liver transplantation for hepatocellular carcinoma following down-staging using sorafenib single therapy. Liver Int 2016;36:1393. [PubMed]
- Na GH, Kim EY, Hong TH, et al. Effects of loco regional treatments before living donor liver transplantation on overall survival and recurrence-free survival in South Korean patients with hepatocellular carcinoma. HPB (Oxford) 2016;18:98-106. [PubMed]
- Giacomoni A, Di Sandro S, Donadon M, et al. Survival after Liver Transplant: Influence of Progression of Disease and of Restoration of the “Milan” Criteria in Patients with Hepato-cellular Carcinoma undergoing Down-staging Procedures. Hepatogastroenterology 2015;62:955-61. [PubMed]
- Lai Q, Avolio AW, Graziadei I, et al. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl 2013;19:1108-18. [PubMed]
- Lee S, Hyuck David Kwon C, Man Kim J, et al. Time of hepatocellular carcinoma recurrence after liver resection and alpha-fetoprotein are important prognostic factors for salvage liver transplantation. Liver Transpl 2014;20:1057-63. [PubMed]
- Wang P, Li H, Shi B, et al. Prognostic factors in patients with recurrent hepatocellular carcinoma treated with salvage liver transplantation: a single-center study. Oncotarget 2016;7:35071-83. [PubMed]
- Samoylova ML, Dodge JL, Yao FY, et al. Time to transplantation as a predictor of hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl 2014;20:937-44. [PubMed]
- Mehta N, Heimbach J, Lee D, et al. Wait Time of <6 and >18 Months Predicts Hepatocellular Carcinoma Recurrence after Liver Transplantation: Proposing a Wait Time “Sweet Spot”. Transplantation 2017. [Epub ahead of print]. [PubMed]
- Schlansky B, Chen Y, Scott DL, et al. Waiting time predicts survival after liver transplantation for hepatocellular carcinoma: a cohort study using the United Network for Organ Sharing registry. Liver Transpl 2014;20:1045-56. [PubMed]
- Halazun KJ, Patzer RE, Rana AA, et al. Standing the test of time: outcomes of a decade of prioritizing patients with hepatocellular carcinoma, results of the UNOS natural geographic experiment. Hepatology 2014;60:1957-62. [PubMed]
- Rich NE, Parikh ND, Singal AG. Hepatocellular Carcinoma and Liver Transplantation: Changing Patterns and Practices. Curr Treat Options Gastroenterol 2017;15:296-304. [PubMed]
- D'Amico F, Schwartz M, Vitale A, et al. Predicting recurrence after liver transplantation in patients with hepatocellular carcinoma exceeding the up-to-seven criteria. Liver Transpl 2009;15:1278-87. [PubMed]
- Gunay Y, Guler N, Yaprak O, et al. Living Donor Liver Transplantation Outcomes for Hepatocellular Carcinoma Beyond Milan or UCSF Criteria. Indian J Surg 2015;77:950-6. [PubMed]
- Wan P, Xia Q, Zhang JJ, et al. Liver transplantation for hepatocellular carcinoma exceeding the Milan criteria: a single-center experience. J Cancer Res Clin Oncol 2014;140:341-8. [PubMed]
- Perea Del Pozo E, Bernal Bellido C, Sendin Matin M, et al. Recurrent Hepatocellular Carcinoma After Liver Transplantation: Analysis of Risk Factors. Transplant Proc 2016;48:2990-3. [PubMed]
- Vasuri F, Malvi D, Rosini F, et al. Revisiting the role of pathological analysis in transarterial chemoembolization-treated hepatocellular carcinoma after transplantation. World J Gastroenterol 2014;20:13538-45. [PubMed]
- Ciccarelli O, Lai Q, Goffette P, et al. Liver transplantation for hepatocellular cancer: UCL experience in 137 adult cirrhotic patients. Alpha-foetoprotein level and locoregional treatment as refined selection criteria. Transpl Int 2012;25:867-75. [PubMed]
- Sapisochin G, Goldaracena N, Laurence JM, et al. The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: A prospective validation study. Hepatology 2016;64:2077-88. [PubMed]
- Aravinthan AD, Bruni SG, Doyle AC, et al. Liver Transplantation is a Preferable Alternative to Palliative Therapy for Selected Patients with Advanced Hepatocellular Carcinoma. Ann Surg Oncol 2017;24:1843-51. [PubMed]
- Grat M, Krasnodebski M, Patkowski W, et al. Relevance of Pre-Transplant alpha-fetoprotein Dynamics in Liver Transplantation for Hepatocellular Cancer. Ann Transplant 2016;21:115-24. [PubMed]
- Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986-94 e3; quiz e14-5.
- Hameed B, Mehta N, Sapisochin G, et al. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl 2014;20:945-51. [PubMed]
- Andreou A, Bahra M, Guel S, et al. Tumor DNA Index and alpha-Fetoprotein Level Define Outcome following Liver Transplantation for Advanced Hepatocellular Carcinoma. Eur Surg Res 2015;55:302-18. [PubMed]
- Grat M, Kornasiewicz O, Lewandowski Z, et al. Combination of morphologic criteria and alpha-fetoprotein in selection of patients with hepatocellular carcinoma for liver transplantation minimizes the problem of posttransplant tumor recurrence. World J Surg 2014;38:2698-707. [PubMed]
- Hong G, Suh KS, Suh SW, et al. Alpha-fetoprotein and (18)F-FDG positron emission tomography predict tumor recurrence better than Milan criteria in living donor liver transplantation. J Hepatol 2016;64:852-9. [PubMed]
- Mailey B, Artinyan A, Khalili J, et al. Evaluation of absolute serum alpha-fetoprotein levels in liver transplant for hepatocellular cancer. Arch Surg 2011;146:26-33. [PubMed]
- Merani S, Majno P, Kneteman NM, et al. The impact of waiting list alpha-fetoprotein changes on the outcome of liver transplant for hepatocellular carcinoma. J Hepatol 2011;55:814-9. [PubMed]
- Boussouar S, Itti E, Lin SJ, et al. Functional imaging of hepatocellular carcinoma using diffusion-weighted MRI and (18)F-FDG PET/CT in patients on waiting-list for liver transplantation. Cancer Imaging 2016;16:4. [PubMed]
- Kornberg A, Freesmeyer M, Barthel E, et al. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transplant 2009;9:592-600. [PubMed]
- Ye YF, Wang W, Wang T, et al. Role of [18F] fludeoxyglucose positron emission tomography in the selection of liver transplantation candidates in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2017;16:257-63. [PubMed]
- Lin CY, Liao CW, Chu LY, et al. Predictive Value of 18F-FDG PET/CT for Vascular Invasion in Patients With Hepatocellular Carcinoma Before Liver Transplantation. Clin Nucl Med 2017;42:e183-e7. [PubMed]
- Ferda J, Ferdova E, Baxa J, et al. The role of 18F-FDG accumulation and arterial enhancement as biomarkers in the assessment of typing, grading and staging of hepatocellular carcinoma using 18F-FDG-PET/CT with integrated dual-phase CT angiography. Anticancer Res 2015;35:2241-6. [PubMed]
- Park MS, Lee KW, Kim H, et al. Primary Living-donor Liver Transplantation Is Not the Optimal Treatment Choice in Patients With Early Hepatocellular Carcinoma With Poor Tumor Biology. Transplant Proc 2017;49:1103-8. [PubMed]
Cite this article as: Viveiros A, Zoller H, Finkenstedt A. Hepatocellular carcinoma: when is liver transplantation oncologically futile? Transl Gastroenterol Hepatol 2017;2:63.


