Metabolic surgery: gastric bypass for the treatment of type 2 diabetes mellitus
Introduction
During the past decades we have been witnesses of the world-increasing pandemic of obesity and type 2 diabetes mellitus (T2DM). Currently, 415 million people worldwide are estimated to have T2DM and if these trends continue, by 2040 some 642 million people, or 1 adult in 10, will have diabetes (1) .The World Health Organization (WHO) estimates that globally, high blood glucose is the third highest risk factor for premature mortality, after high blood pressure and tobacco use; therefore, preventing cardiovascular complications focusing in glucose control, lipids and blood pressure is necessary (2).
Conventional management for T2DM include lifestyle interventions and medication (3), but despite the increasing number and variety of antidiabetic drugs that have emerged during the last two decades to achieve optimal glycemic control, T2DM control remains suboptimal (4,5). Current treatment goals proposed by American Diabetes Association for T2DM are glycated hemoglobin (A1c) <7%, low-density lipoprotein cholesterol levels <100 mg/dL and blood pressure <130–80 mmHg (3,6), and less than 20% of USA patients achieve these levels in triple target despite having the best medical treatment.
Bariatric surgery was first described just for reducing weight in severely obese patients. A Swedish Obese Subjects (SOS) study, demonstrated that the surgery group arm not only had more drastic and sustainable average reduction of excess body weight but also had remarkable beneficial effects on cardiovascular risk factors, such as waist circumference, blood pressure, glucose and insulin levels, uric acid, triglyceride and HDL cholesterol levels when compared with conventionally treated patients (7-10). Even though the SOS study showed a reduction of the number of cardiovascular events and overall mortality in the surgery group (HR 0.76, CI 95%) (11,12), one of the most relevant points was the finding of absence of significant relationship between cardiovascular mortality and body mass index (BMI) (7,13).
Gastrointestinal (GI) operations have demonstrated, especially those that involve food rerouting through the GI tract that are safe and provide better outcomes for weight loss and improvement in glucose metabolism in obese patients with T2DM in comparison to conventional strategies.
Several trials have demonstrated that surgical procedures improve glycemia independently of weight loss (14,15), and also have a positive impact on lipids profile and blood pressure control, with very good long-term effects on cardiovascular morbidity and mortality (5,11,16,17).
Better glycemic control was seen in patients following a Roux-en-Y gastric bypass (RYGB) comparing them with patients with optimal lifestyle and pharmacological interventions (18,19).
The way how these operations control T2DM have become the subject of intense research, fueled by experimental evidence that GI bypass surgery can induce a very rapid diabetes-enhancing effect, independent of weight loss, making it a promising tool to treat T2DM (5,20).
In this way, in 2017 the American Diabetes Association’s recommendations on metabolic surgery for adult patients with type 2 diabetes have been revised (1). The “Standards of Medical Care” 2017 have expanded the indications for metabolic surgery to include patients with inadequately controlled type 2 diabetes who have a BMI as low as 30 kg/m2 (27.5 kg/m2 in Asian Americans). They declared in the Standards that metabolic surgery “should be recommended” in T2DM with BMI >40 kg/m2 (BMI >37.5 in Asian Americans) regardless of glycemic control achieved, and in adults with BMI 35–39.9 kg/m2 (32.5–37.4 kg/m2 in Asian Americans) when the glycemic level is not adequately controlled despite using the best available medical treatment. They say that “metabolic surgery should be considered” for adults with T2DM and BMI 30–34.9 kg/m2 if there is no control of the glycemia with medical treatment (6,8,9).
Mechanisms related to improvement in glucose homeostasis
Improvement in glucose homeostasis after a gastric bypass begins short days after surgery, before significant weight loss occurs. The important role of the GI tract in the regulation of glucose metabolism was firmly established along the past two decades. However, it seems that the physiological and molecular mechanisms underlying glycemic improvement after metabolic surgery still remains incompletely understood. Several mechanisms have been proposed since Pories et al. published “Is Type II Diabetes Mellitus a Surgical Disease?” and “Who Would Have Though It? An Operation Proves to Be the Most Effective Therapy For Adult Onset Diabetes Mellitus”. In that last publication, Pories et al. suggested that caloric restriction was the cornerstone for glycemic improvement with collaboration of the nutrient exclusion of the proximal gut and rapid delivery of them to the distal gut. In parallel, several studies demonstrated that changes in gut hormones, bile acids metabolism, GI tract nutrient sensing, and microbiome are implicated in the effects of RYGBP for T2DM. Rubino et al. showed that GI surgical procedures that involve food rerouting were related to weight independent antidiabetes effect of surgery by improving both, insulin sensitive and production (21,22). RYGB increase the delivery rate of nutrients into the small intestine, with a fast glycemic rise and increased secretion of GLP-1, restoring first-phase insulin response (22). This leads to an improved insulin secretion after a meal, suggesting enhancement B-cell function and increase of B-cell mass.
There exist numerous mechanism proposed to explain how RYGB exerts its effects on B-cell islets. The strongest hypothesis is that gut hormone changes after RYGB are probably the principal mechanism involved in rapid and weight independent anti-diabetic effect of surgery. Indeed, the incretin effect, a hormonal response of B cells to exacerbate insulin secretion after an oral load of glucose mediated by GLP-1—an intestinal hormone released by L cell in the ileum—appears to be enhanced after surgery. In fact, surgery causes a three to fourfold increase levels of GLP-1 (23) resulting in further stimulation to insulin release from the pancreas as well as an antiapoptotic effect on the B-cell.
On the other side, the anti-incretin theory postulates that the uncertain effect (GLP-1, GIP) nutrient passage through the small bowel could also activate “anti-incretin” or feedback mechanisms to balance the actions of GLP-1 and GIP such us enhance insulin secretion. In the absence of one or more of these feedback mechanisms, these effects would expose to exacerbated insulin hypersecretion and B-cell function and growth, with the concomitant risk of postprandial hyperinsulinemic hypoglycemia and uncontrolled B-cell proliferation. Reduction of nutrient passage and stimuli on the gut by surgical procedures that exclude parts of the foregut and the arrival of them to the handgun could restore appropriate incretin/anti-incretin balance explaining improvement of T2DM (24-30). Bile acids are now considered to be important regulators of energy balance and metabolism, primarily via de nuclear farnesoid X receptor (FXR) and the G-protein-coupled receptor (TGR5). Several trials demonstrated that the duodenum and jejunum sense nutrients and initiate a feedback response through the gut-brain-liver axis to regulate hepatic glucose production. When nutrients are delivered to medium jejunum led a greater insulin sensitivity for glucose and fatty acids (20,26,31-39).
Eligibility criteria for metabolic surgery
Bariatric and metabolic surgery might be misunderstood as synonymous, but they are not since bariatric operation primary indication is to achieve weight loss in morbidly obese patients, and the metabolic benefits (improvement in T2DM, hypertension, dyslipidemia) and reduction of overall mortality might be considered beneficial side effects (40).
In 1978, Varco and Buchwald defined “Metabolic surgery: manipulation of an organ system to achieve a biological result for a potential health gain” (41). This definition involves the notion of an anatomical and functional procedure on a normal GI tract aimed at reducing or reverting an altered function that cause a metabolic disease (42).
GI surgery for the specific intention to treat T2DM was first recommended at the 2007 Diabetes Surgery Summit (DSS) (43) and implicated a shift in the primary focus of surgery from mere weight management to treatment or improvement of metabolic illness may have significant and practical ramification (40,44,45).
The preoperative evaluation for metabolic surgery should include clinical considerations such as inadequate response of glycemia to optimal medical therapy or the presence of other cardiovascular risk factors. In fact, the SOS study demonstrated that baseline BMI does not predict the benefits of surgery related to T2DM development, cancer, cardiovascular events or death (39,40) but surgical benefits were notably predicted by high baseline insulin and/or glucose levels, presumably reflecting insulin resistance. In this background, BMI cut-off at 35 kg/m2 is not an accurate parameter to predict the potential of surgery to induce glycemic and metabolic control, so it should not be considered a stand-alone criterion for surgical selection (43).
Results of metabolic surgery and prognostic factors
Metabolic surgery is a highly effective treatment of T2DM, inducing improvement and/or remission in obese patients, decreases the risk of macrovascular complications, prevents or delays microvascular complications and improves survival (14,15,18).
The GI tract is an important contributor to normal glucose homeostasis (41) and increasing evidence has demonstrated that GI operations can dramatically ameliorate or prevent T2DM. Beyond inducing weight loss-related metabolic improvements, some operations engage mechanisms that improve glucose homeostasis independent of weight loss. Moreover, when compared medical therapy (conventional and/or intensive) for T2DM versus surgical GI procedures, surgery resulted in significantly better glucose control than did medical therapy alone; so it’s not surprising that bariatric-metabolic surgery is being used throughout the world to treat diabetes (42).
Defining the idea of T2DM remission following surgery has been controversial (40,41,45).
Various factors have been proposed as predictor of remission T2DM after RYGB (40). Durable remission has been seen early stage of T2DM (41), loss of a large percentage of excess bodyweight, young age and low BMI (25–35 kg/m2). Duration of diabetes, usage of insulin and duration of insulin usage have all been reported to negatively predict the likelihood of diabetes remission after surgery (39,40).
The DiaRem scoring system is a method that uses four preoperative clinical variables (use of insulin before surgery, age, HbA1c and diabetes drugs before surgery) to predict the probability of remission of T2DM after RYGB surgery. It predicts remission irrespective of early or late occurrence, and includes patients in partial remission who might be progressing to complete remission (45).
However, the mayor benefit of surgery, would probably not be to improve glycemic control per se but rather to reduce microvascular and macrovascular complications associated with T2DM (40), so in the selection of T2DM patients for metabolic surgery should be considered a balance between the diabetes-related risk of micro and macrovascular complications and the potential benefits of surgery rather than BMI solely (41,45).
The STAMPEDE (Surgical Therapy And Medications Potentially Eradicate Diabetes Efficiently) trial is the largest randomized trial with one of the longest follow-ups comparing medical therapy with bariatric surgery (3). In the 5-year follow-up report from the STAMPEDE trial, the authors shows that bariatric surgery’s beneficial effects on blood glucose control in mild and moderately obese patients with type 2 diabetes may persist for up to five years, with the advantage over diabetes medications. The 5-year follow-up also reported that:
- Over 88% of RYGBP and sleeve gastrectomy patients maintained healthy blood glucose levels without the use of insulin;
- There are 29% of RYGBP patients and 23% of sleeve gastrectomy patients achieved normal blood glucose levels, compared to just 5% of those on medication alone;
- Weight loss was significantly greater with gastric RYGBP and sleeve gastrectomy than with medications and was the primary driver for glucose control.
Finally, metabolic surgery should be considered as a broad surgical specialty where the GI operations are used with the primary intent to treat diabetes and metabolic disease. This definition is not based on the assumption of whether the site of surgery is normal or pathological, yet it is consistent with the evidence that GI procedures engage mechanisms of action beyond the mechanical restriction of energy intake and altered nutrient absorption. According to such definition, standard Roux-en-Y gastric bypass should be thought as a “metabolic” rather than “bariatric” surgery (6,9,14,15,40).
Surgical technique
In the last 10 years, multiple publications demonstrated improvement of blood glucose levels and concomitant reduction of morbidity and mortality related to CV events after BS/MS (26-29). RYGB technique was the first technique that proved its effectiveness in remission of T2DM in morbidly obese patients. Laparoscopic RYGB is the most accepted and effective procedure; it is also the safest and has the best long term T2DM remission rates compared with other restrictive techniques. These characteristics postulate laparoscopic RYGB as the gold standard (30,31).
RYGB technique description
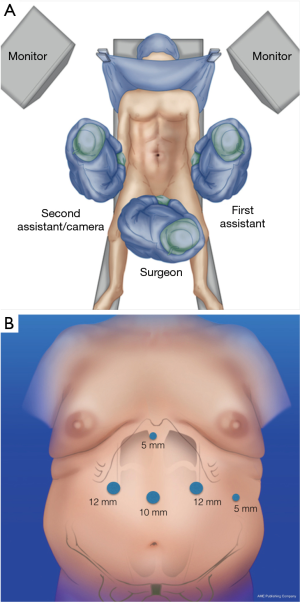
The patient is in a split legs position and the surgeon is located in between the patient’s legs. Abdominal insufflation is performed at 14 mmHg was obtained with a Veress needle placed through the umbilicus of the patient (27,33-37). Trocar placement: 10 mm in the midline (between xyphoid and umbilicus); two of 12 mm, one in the right upper quadrant and a left one in the mid-clavicular line; after that two more 5-mm trocars were used, one for liver retraction just distal to the xyphoid; and the other 5-mm of distal to the costal margin (Figure 1A,B).
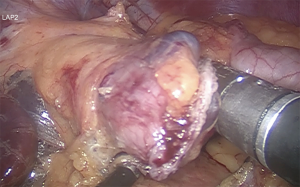
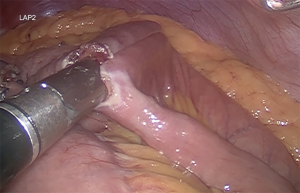
Gastric pouch of approximate volume 30 to 50 mL, calibrated with 36 Fr Boogies. The approximated size should be about 4×7 cm. The stomach is transected firing blue load linear staplers, to disconnect the small upper stomach from the remaining portion of the stomach (Figure 2) (27,38,39).
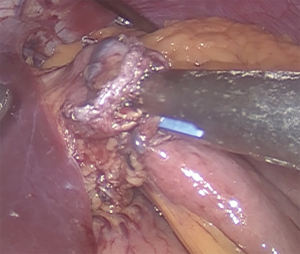
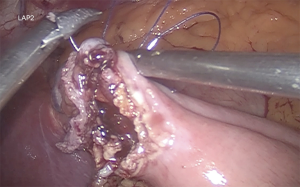
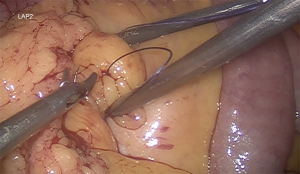
Gastrojejunostomy is recommended a hybrid technique (manual-mechanic), although it can be performed fully hand sewn. It should be always calibrated with a 34–36 Fr boogie. The omentum is divided. The gastrojejunal anastomosis is about to be performed (5). For this step, a blue load linear stapler is inserted only half way into the pouch hole in order to create an anastomosis that is about 2 cm in length before firing. This anastomosis is 2 cm in diameter (Figure 3). A running suture is used to close the defect (Figure 4) (27,39).
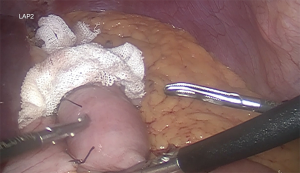
It is recommended a biliary limb of 100-cm of length from duodenojejunal flexure; a 150-cm alimentary limb: from gastrojejunostomy and a jejunojejunostomy with hybrid mechanic-manual techniques (Figure 5). It’s also suggested the antecolic/antegastric loop ascent to decrease the risk of vowel obstruction. It is advisable the closure of mesenteric gaps, Petersen’s and intermesenteric spaces to decrease the risk of internal hernia (Figure 6). Blue test is performed. Finally a last stapler is fired in the jejunum (Figure 7). Finally, the abdominal cavity is drained with a Jackson-Pratt (JP) drain for about 7 days (27,39).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- IDF Atlas 7th Edition, 2015. Available online: http://www.diabetesatlas.org
- Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580-91. [Crossref] [PubMed]
- American Diabetes Association Standards of Medical Care in Diabetes—2016. Diabetes Care. Available online: http://care.diabetesjournals.org/content/suppl/2015/12/21/39.Supplement_1.DC2/2016-Standards-of-Care.pdf
- Stark Casagrande S, Fradkin JE, Saydah SH, et al. The prevalence of meeting a1C, blood pressure, and LDL goals among People with diabetes, 1988-2010. Diabetes Care 2013;36:2271-9. [Crossref]
- Cohen R, Caravatto PP, Petry T. Surgery for Diabetes. Curr Surg Rep 2013;1:160-6. [Crossref]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood glucose control with sulfonylureas or insulin compared with conventional treatment and risk for complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53. [Crossref] [PubMed]
- Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419-30. [Crossref] [PubMed]
- ADVANCE Collaborative Group, Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560-72. [Crossref] [PubMed]
- Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129-39. [Crossref] [PubMed]
- Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683-93. [Crossref] [PubMed]
- Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012;307:56-65. [Crossref] [PubMed]
- Sjöström L, Narbro K, Sjostrom D, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741-52. [Crossref] [PubMed]
- Cohen R, Caravatto PP, Petry T. Metabolic Surgery for Type 2 Diabetes in Patients with a BMI of <35 kg/m(2): A Surgeon's Perspective. Obes Surg 2013;23:809-18. [Crossref] [PubMed]
- Mingrone G., Panunzi S, De Gaetano A, et al. Bariatric Surgery versus conventional medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1577-85. [Crossref] [PubMed]
- Schauer PR, Kashyap SR., Wolski K, et al. Bariatric Sugery versus intensive medical therapy in obese patients with diabetes. N ENgl J Med 2012;366:1567-76. [Crossref] [PubMed]
- Cohen R, Caravatto PP, Petry T, et al. Role of metabolic surgery in less obese or non-obese subjects with type 2 diabetes: influence over cardiovascular events. Curr Atheroscler Rep 2013;15:355. [Crossref] [PubMed]
- Cummings DE, Flum DR. Gastrointestinal surgery as a treatment for diabetes. JAMA 2008;299:341-3. [Crossref] [PubMed]
- Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309:2240-9. [Crossref] [PubMed]
- Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 2008;93:2479-85. [Crossref] [PubMed]
- Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg 2004;239:1-11. [Crossref] [PubMed]
- Bojsen-Møller KN, Dirksen C, Jørgensen NB, et al. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes 2014;63:1725-37. [Crossref] [PubMed]
- Salinari S, Bertuzzi A, Guidone C, et al. Insulin sensitivity and secretion changes after gastric by pass in normotolerant and diabetic obese subjects. Ann Surg 2013;257:462-8. [Crossref] [PubMed]
- Dirksen C, Bojsen-Møller KN, Jørgensen NB, et al. Exaggerated release and preserved insulinotropic action of glucagon-like peptide-1 underlie insulin hypersecretion in glucose-tolerant individuals after Roux-en-Y gastric bypass. Diabetologia 2013;56:2679-87. [Crossref] [PubMed]
- Lindqvist A, Spegel P, Ekelund M, et al. Gastric by pass improves B-cell function and increases B-cell mass in a porcine model. Diabetes 2014;63:1665-71. [Crossref] [PubMed]
- Rubino F, Amiel SA. Is the gut the “sweet spot” for the treatment of diabetes? Diabetes 2014;63:2225-8. [Crossref] [PubMed]
- Jacob BP, Gagner M. New developments in gastric bypass procedures and physiological mechanisms. Surg Technol Int 2003;11:119-26. [PubMed]
- Palermo M, Gimenez M, Gagner M. Laparoscopic gastrointestinal surgery. Novel techniques, extending the limits. Editorial AMOLCA, 2014.
- Himpens JM. The gastrojejunostomy in laparoscopic Roux-en-Y gastric bypass. Semin Laparosc Surg 2004;11:171-7. [PubMed]
- Cadière GB, Himpens J, Dapri G. Laparoscopic stomach bypass surgery. Chirurg 2005;76:668-77. [PubMed]
- Palermo M, Dapri G. Single-Port Laparoscopic Surgery. Cdn.intechopen.com 2014;17:S191.
- Collins BJ, Miyashita T, Schweitzer M, et al. Gastric bypass: why Roux-en-Y? A review of experimental data. Arch Surg 2007;142:1000-3; discussion 1004. [Crossref] [PubMed]
- Madan AK, Harper JL, Tichansky DS. Techniques of laparoscopic gastric bypass: on-line survey of American Society for Bariatric Surgery practicing surgeons. Surg Obes Relat Dis 2008;4:166-72; discussion 172-3. [Crossref] [PubMed]
- Franco JV, Ruiz PA, Palermo M, et al. A review of studies comparing three laparoscopic procedures in bariatric surgery: sleeve gastrectomy, Roux-en-Y gastric bypass and adjustable gastric banding. Obes Surg 2011;21:1458-68. [Crossref] [PubMed]
- Acquafresca PA, Palermo M, Duza GE, et al. Gastric Bypass versus Sleeve gastrectomy: comparison between type 2 Diabetes weight loss and complications. Review of randomized control trails. Acta Gastroenterol Latinoam 2015;45:143-54. [PubMed]
- Acquafresca PA, Palermo M, Rogula T, et al. Early Complications After Gastric By-Pass: a literature review. Arq Bras Cir Dig 2015;28:74-80. [Crossref] [PubMed]
- Alfonso Torquati et al. Comparative studies an metabolic effects of sleeve gastrectomy. Duke Surgery.
- Busetto L, Dixon J, De Luca M, et al. Bariatric surgery in class I obesity: a Position Statement from the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO). Obes Surg 2014;24:487-519. [Crossref] [PubMed]
- Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248-56.e5. [Crossref] [PubMed]
- Ramos AC, Silva AC, Ramos MG, et al. Simplified gastric bypass: 13 years of experience and 12,000 patients operated. Arq Bras Cir Dig 2014;27 Suppl 1:2-8. [Crossref] [PubMed]
- Rubino F, Nathan DM, Eckel RH, et al. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Surg Obes Relat Dis 2016;12:1144-62. [Crossref] [PubMed]
- Buchwald H. Metabolic Surgery. In: Lucchese M, Scopinaro N. editors. Minimally Invasive Bariatric and Metabolic Surgery. New York: Springer, 1978:(69-79).
- De Luca M, Angrisani L, Himpens J, et al. Indications for Surgery for Obesity and Weight- related Diseases: Position Statements from the International Federation for the Surgery of Obesity an Metabolic Disorders (IFSO). Obes Surg 2016;26:1659-96. [Crossref] [PubMed]
- Rubino F, Kaplan LM, Schauer PR, et al. The Diabetes Surgery Summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg 2010;251:399-405. [Crossref] [PubMed]
- Rubino F, Shukla A, Pomp A, et al. Bariatric, metabolic, and diabetes surgery: what's in a name? Ann Surg 2014;259:117-22. [Crossref] [PubMed]
- Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367:695-704. [Crossref] [PubMed]
Cite this article as: Quevedo Md, Palermo M, Serra E, Ackermann MA. Metabolic surgery: gastric bypass for the treatment of type 2 diabetes mellitus. Transl Gastroenterol Hepatol 2017;2:58.