Robotic gastrectomy for gastric cancer
Introduction
Since the first case of laparoscopic gastrectomy (LG) was reported in 1994, it has been increasingly performed all over the world (1). Many potential benefits of the procedure, such as less bleeding, early recovery, and good cosmetic results, compared with conventional open gastrectomy (OG) have been reported, and the safety of the procedure was confirmed by randomized trials conducted in Asia (2-8). Equivalent long-term survival outcomes in patients with early gastric cancer were also reported in a randomized control trial (9). Consequently, LG is generally regarded as one of the standard treatments for early gastric cancer, and it is expected that the indications for LG will expand further to advanced gastric cancer (10).
However, LG has several drawbacks. Limitation in the movement range of forceps and the two-dimensional surgical view available to operating surgeons in LG, though recent technological advancements have facilitated this to some degree, have been serious shortcomings of the procedure. Robotic gastrectomy (RG) may enable us to overcome these shortcomings.
Using the da Vinci® Surgical System (Intuitive Surgical, Sunnyvale, CA, USA), surgeons were able to attain a three-dimensional surgical view, instrument flexibility, tremor suppression, and improved ergonomics (11-14). The number of RG performed per year has been increasing, particularly in East Asia where the incidence of gastric cancer is high and approximately half of the cases are diagnosed as early gastric cancer. In this paper, we reviewed the literature about RG for gastric cancer to confirm the current standpoint and assess future perspectives.
Absolute benefit of RG over LG
Both RG and LG are generally classified as minimally invasive surgeries (MIS) and the surgical procedure itself is quite similar between them. The largest and most important difference is that articulated devices are only available in RG. With articulated devices, surgeons are able to perform every procedure more meticulously, which can result in less bleeding and damage to organs. The tremor suppression function is also helpful to keep a stable surgical field and effective to reduce organ injury. Another advantage of RG is its fine three-dimensional image. Although a three-dimensional image has become available in LG with special equipment, it is not yet commonly used. Articulated devices and three-dimensional images are a potential benefit of RG and could facilitate each procedure dramatically, particularly such difficult procedures as hand suturing in deep surgical fields and extensive lymphadenectomy.
Single arm studies
There are many single arm retrospective and prospective cohort studies, mostly from Asia and Italy (Table 1). Among them, Park et al. included the largest number with 200 patients, which included their initial cases (25). The aim of most retrospective single-arm studies was to confirm the safety of RG by evaluating early surgical outcomes, such as morbidity and mortality rate, estimated intraoperative blood loss, and operation time. Although the morbidity rate ranged widely from 0% to 41.1%, probably due to the limited number of cases in each study and different indications for RG among studies, most authors concluded that RG is feasible in terms of safety. The mortality rates were quite low, with less than 1% mortality reported in most series.
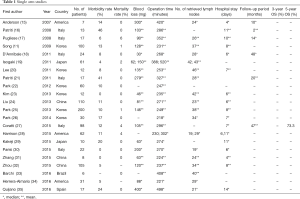
Full table
Some novel approaches were reported. Zhang et al. reported transvaginal specimen extraction after RG (31). He reported eight successful cases, and this procedure will lead to much better cosmetic results although it should be evaluated in the future with a larger number of patients. Other unique reports include RG with near-infrared fluorescence imaging, and comparison between RG with and without ultrasonic shears (29,34).
Although single arm studies are informative and very important in the early development stage of new procedures, the feasibility of RG has already been reported in a number of case series. This feasibility should be confirmed by comparative studies, ideally by prospective randomized trials, for RG to be accepted widely.
Retrospective comparative studies
Quite a few comparative studies exist, and most are from Asian countries (Table S1). Although the number of RGs in each study is limited, Kim et al. featured the largest number of cases, which included 4,542 OG, 881 LG, and 436 RG (36). As in retrospective single arm studies, authors generally evaluated early surgical outcomes between the groups, including surgical morbidity and mortality, estimated blood loss, operation time, the number of harvested lymph nodes, and postoperative hospital stay.
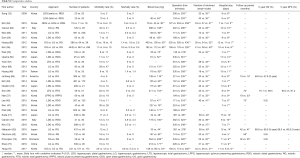
Full table
Although some reported a lower incidence of postoperative morbidity in RG, it was equivalent between the groups in most reports, and mortality rates were not significantly different in any of the reports. Among the studies, similar trends were observed, which included relatively less estimated blood loss and longer operation time following RG than LG, equivalent number of harvested lymph nodes and similar length of postoperative hospital stay between RG and LG. Considering the results of these retrospective comparative studies, RG seems to be as feasible as LG and OG in terms of early surgical outcomes, but the comparability of the procedures is still uncertain, and selection bias might have affected results. To eliminate probable selection biases, Han et al. selected patients by propensity score matching, and compared surgical outcomes between laparoscopic and robotic pylorus preserving gastrectomies (37). They found that robotic procedures took approximately one extra hour compared with the laparoscopic approach, but other parameters such as postoperative hospital stay, surgical morbidity and mortality were not significantly different.
Although equivalent surgical outcomes have been reported, superiority of the robotic approach to other approaches was rarely shown in retrospective studies. Some reported the potential benefit of RG in more complicated procedures such as total gastrectomy. Park et al. reported the potential benefit of RG for patients undergoing TG with D2 lymphadenectomy (38). Son et al. compared surgical outcomes between 58 patients undergoing laparoscopic and 51 patients undergoing robotic spleen preserving D2 total gastrectomy, and found almost equivalent surgical outcomes with higher numbers of retrieved lymph nodes along the splenic artery after robotic surgery (39). Surgery for obese patients requires a more sophisticated approach and RG could be beneficial, but the potential benefit of RG for these patients is still controversial (38,40).
Seo et al. paid special attention to the superiority of RG in terms of the incidence of postoperative pancreas fistula, and reported a lower incidence of postoperative pancreas fistula in RG than LG (41). Their multivariable analysis also identified the robotic approach as being associated with a lower incidence of POPF compared to the laparoscopic approach, indicating that meticulous procedures with articulated devices could reduce pancreas damage during lymph node dissection of the suprapancreatic area.
Okumura et al. compared short and long term survival outcomes of elderly patients undergoing RG and LG, and found no significant difference between the groups (42). The feasibility of RG for elderly patients is also important for future widespread adoption of this procedure, considering the increasing expected age of the general population.
Apparently, articulated devices with tremor suppression function facilitate surgeries from the surgeons’ viewpoint, but it is a different question whether RG is actually beneficial for patients, and this needs to be clarified. Only marginal differences favoring RG over LG were shown in some retrospective comparative studies, and superiority needs to be confirmed by well designed comparative studies for RG to be accepted more widely.
Prospective studies
The number of prospective studies is extremely limited so far. Tokunaga et al. reported results of early and late phase II studies, in which 20 and 123 patients were included, respectively, and the incidence of intra-abdominal infectious complication was set as a primary endpoint (43,44). In their early phase II study, the incidence of intra-abdominal infectious complication was 0%, and it was 3.3% in their late phase II study. In both studies, the null hypothesis was rejected, and it was concluded that RG is a feasible procedure in terms of safety.
In Korea, a prospective non-randomized comparative study was conducted (45). In the study, a total of 423 patients selected either RG or LG after they received a comprehensive explanation of each procedure, and were matched according to surgeon, extent of gastric resection, and sex. Per-protocol analysis (185 patients in each group) showed similar early surgical outcomes including morbidity and mortality rate, except for longer operation time in the RG group. They also reported significantly higher total cost in the RG group (US$13,432) than the LG group (US$8,090). Park et al. conducted a subset analyses of this study, and found that RG may be beneficial for patients undergoing D2 lymphadenectomy, although they failed to show the benefit of RG in patients undergoing total gastrectomy or those with obesity (46).
The results of a single-center prospective randomized trial were reported by Wang et al. (47) They randomized a total of 311 patients to either open (n=153) or robotic (n=158) gastrectomy groups by the envelope method. They showed similar complication rates between the groups, and less estimated blood loss, longer duration of surgery, and shorter postoperative hospital stay in the robotic group than open group.
Learning curve
Many surgeons have focused on the learning curve effects in RG, and hypothesized that less experience would be necessary to reach the plateau of the learning curve in RG than in LG (22,25,32,48-52). Kim et al. compared the learning curve effect in RG and LG, and reported that experience with LG could affect the learning process in RG (49). Provided that an experienced laparoscopic surgeon begins RG, fewer cases of RG are necessary to reach steady status, and satisfactory surgical outcomes could be obtained.
Quality of life assessment
Park et al. assessed chronological change in health-related quality of life (HRQOL) after RG using the European Organization for Research and Treatment of Cancer (EO-RTC) core questionnaire (QTC-C30) and the gastric cancer-specific module (QLQ-STO22). They compared the HRQOL of patients undergoing RG with that of the general population. Although immediate deterioration of HRQOL after RG was shown, it recovered to baseline level within 3 months and was maintained for at least 1 year (26).
Cost analysis
Because RG requires an expensive machine and devices, cost effectiveness is another intriguing issue for surgeons. In Korea and Japan, where more than half of reports have been published, the cost for RG is not reimbursed by the governments so far, and therefore patients or hospitals have to pay additional fees (53). This is a possible reason why randomized trials have not been conducted in either country.
Although the number of reports is limited, some compared medical expenses between RG and LG, and reported that RG required approximately twice as much medical expense as LG (48,54-56). It is expected that medical expenses associated with RG will decrease in the future.
Oncological outcomes
Although most reports in the literature have focused on early surgical outcomes, some have investigated long-term oncological outcomes after RG, and compared them with those of LG. Equivalent oncological outcomes have been reported, although selection bias must be taken into account and comparability assessed carefully. Median follow-up periods in RG studies have been relatively short, except for several Korean series with 5-year or longer median follow-ups (39,40,42), and we cannot obtain any conclusive results from these retrospective studies in terms of oncological long-term outcomes of RG. The Korean prospective comparative study may shed light on this issue, although it is not a randomized trial (45). Considering the total medical expense of RG, long-term outcomes need to be better than those of LG, and should be confirmed by future prospective trials.
Discussion
So far, there has been no multicenter randomized controlled trial investigating the feasibility of RG. It seems to be safer than or as safe as LG, considering the results of studies included in this review, although its oncological safety is not confirmed and this should be clarified in the future.
Even if RG is actually safer than LG, it is an expensive procedure, and this issue should be resolved for RG to be accepted as a standard treatment in the future (45,48,54-56). Considering the extremely high cost required for RG, a marginal benefit in early surgical outcomes would not be enough. Instead, clearly better early or long-term survival outcomes would be necessary to outweigh the cost issue. The other likely scenario, which is more plausible, is that the advancement of technology will offer us a more reasonably priced robotic system. With a cost-effective system, a marginal benefit in early surgical outcomes might be enough for RG to be accepted as one of the standard treatment options.
Lack of evidence in terms of oncological safety is another problem for RG. Although some have reported feasible long-term survival after RG, the number of patients in each series is too small to obtain any conclusive results (39,40,42). In addition, because survival data is available only from retrospective studies, potential bias could not be eliminated. Surgeons should think about this issue more seriously when they operate on patients with advanced gastric cancer.
Current standard treatment for advanced gastric cancer is D2 gastrectomy followed by adjuvant chemotherapy in Asia, and perioperative chemotherapy with D2 gastrectomy in Europe (57-60). Therefore, a surgical procedure with less postoperative morbidity is very important in order to start adjuvant chemotherapy without delay. If it is true that the incidence of postoperative complication is lower after RG than LG thanks to more meticulous procedures with articulated forceps, patients receiving RG might be able to receive adjuvant chemotherapy earlier and dose intensity might be higher, possibly resulting in improved long-term survival. In addition, considering the recently reported relationship between severe intra-abdominal infectious complication and poor long-term survival, RG could improve long-term survival by reducing postoperative complications (61,62).
In summary, there are a number of reports showing the feasibility of RG by either single arm or comparative studies. However, there is no solid evidence for RG due to the lack of multicenter randomized clinical trials. Considering the higher medical expenses associated with RG, its superiority in terms of long-term survival outcomes needs to be confirmed in the future for it to be accepted more widely.
Acknowledgements
None
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994;4:146-8. [PubMed]
- Memon MA, Khan S, Yunus RM, et al. Meta-analysis of laparoscopic and open distal gastrectomy for gastric carcinoma. Surg Endosc 2008;22:1781-9. [Crossref] [PubMed]
- Kawamura H, Yokota R, Homma S, et al. Comparison of respiratory function recovery in the early phase after laparoscopy-assisted gastrectomy and open gastrectomy. Surg Endosc 2010;24:2739-42. [Crossref] [PubMed]
- Ohtani H, Tamamori Y, Noguchi K, et al. A meta-analysis of randomized controlled trials that compared laparoscopy-assisted and open distal gastrectomy for early gastric cancer. J Gastrointest Surg 2010;14:958-64. [Crossref] [PubMed]
- Kim YW, Baik YH, Yun YH, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg 2008;248:721-7. [Crossref] [PubMed]
- Kodera Y, Fujiwara M, Ohashi N, et al. Laparoscopic surgery for gastric cancer: a collective review with meta-analysis of randomized trials. J Am Coll Surg 2010;211:677-86. [Crossref] [PubMed]
- Takagi M, Katai H, Mizusawa J, et al. A phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer (JCOG0912): Analysis of the sfety and short-term clinical outcomes. J Clin Oncol 2015;33:abstr 4017.
- Kim W, Kim HH, Han SU, et al. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared With Open Distal Gastrectomy for Stage I Gastric Cancer: Short-term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg 2016;263:28-35. [Crossref] [PubMed]
- Kim HH, Han SU, Kim MC, et al. Long term outcomes of laparoscopic distal gastrectomy compared with open distal gastrectomy for clinical stage I gastric adenocarcinoma (KLASS-01): a multi-center prospective randomized controlled trial. J Clin Oncol 2016;34:abstr 4060.
- Hu Y, Huang C, Sun Y, et al. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol 2016;34:1350-7. [Crossref] [PubMed]
- Song J, Oh SJ, Kang WH, et al. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg 2009;249:927-32. [Crossref] [PubMed]
- Song J, Kang WH, Oh SJ, et al. Role of robotic gastrectomy using da Vinci system compared with laparoscopic gastrectomy: initial experience of 20 consecutive cases. Surg Endosc 2009;23:1204-11. [Crossref] [PubMed]
- Hashizume M, Sugimachi K. Robot-assisted gastric surgery. Surg Clin North Am 2003;83:1429-44. [Crossref] [PubMed]
- Kakeji Y, Konishi K, Ieiri S, et al. Robotic laparoscopic distal gastrectomy: a comparison of the da Vinci and Zeus systems. Int J Med Robot 2006;2:299-304. [Crossref] [PubMed]
- Anderson C, Ellenhorn J, Hellan M, et al. Pilot series of robot-assisted laparoscopic subtotal gastrectomy with extended lymphadenectomy for gastric cancer. Surg Endosc 2007;21:1662-6. [Crossref] [PubMed]
- Patriti A, Ceccarelli G, Bellochi R, et al. Robot-assisted laparoscopic total and partial gastric resection with D2 lymph node dissection for adenocarcinoma. Surg Endosc 2008;22:2753-60. [Crossref] [PubMed]
- Pugliese R, Maggioni D, Sansonna F, et al. Robot-assisted laparoscopic gastrectomy with D2 dissection for adenocarcinoma: initial experience with 17 patients. J Robot Surg 2008;2:217-22. [Crossref] [PubMed]
- D'Annibale A, Pende V, Pernazza G, et al. Full robotic gastrectomy with extended (D2) lymphadenectomy for gastric cancer: surgical technique and preliminary results. J Surg Res 2011;166:e113-20. [Crossref] [PubMed]
- Isogaki J, Haruta S, Man IM, et al. Robot-assisted surgery for gastric cancer: experience at our institute. Pathobiology 2011;78:328-33. [Crossref] [PubMed]
- Lee HH, Hur H, Jung H, et al. Robot-assisted distal gastrectomy for gastric cancer: initial experience. Am J Surg 2011;201:841-5. [Crossref] [PubMed]
- Patriti A, Ceccarelli G, Ceribelli C, et al. Robot-assisted laparoscopic management of cardia carcinoma according to Siewert recommendations. Int J Med Robot 2011;7:170-7. [Crossref] [PubMed]
- Park SS, Kim MC, Park MS, et al. Rapid adaptation of robotic gastrectomy for gastric cancer by experienced laparoscopic surgeons. Surg Endosc 2012;26:60-7. [Crossref] [PubMed]
- Kim YM, Baek SE, Lim JS, et al. Clinical application of image-enhanced minimally invasive robotic surgery for gastric cancer: a prospective observational study. J Gastrointest Surg 2013;17:304-12. [Crossref] [PubMed]
- Liu XX, Jiang ZW, Chen P, et al. Full robot-assisted gastrectomy with intracorporeal robot-sewn anastomosis produces satisfying outcomes. World J Gastroenterol 2013;19:6427-37. [Crossref] [PubMed]
- Park JY, Kim YW, Ryu KW, et al. Emerging Role of Robot-assisted Gastrectomy: Analysis of Consecutive 200 Cases. J Gastric Cancer 2013;13:255-62. [Crossref] [PubMed]
- Park JY, Eom BW, Jo MJ, et al. Health-related quality of life after robot-assisted distal gastrectomy in early gastric cancer. World J Surg 2014;38:1112-20. [Crossref] [PubMed]
- Coratti A, Fernandes E, Lombardi A, et al. Robot-assisted surgery for gastric carcinoma: Five years follow-up and beyond: A single western center experience and long-term oncological outcomes. Eur J Surg Oncol 2015;41:1106-13. [Crossref] [PubMed]
- Harrison LE, Yiengpruksawan A, Patel J, et al. Robotic gastrectomy and esophagogastrectomy: A single center experience of 105 cases. J Surg Oncol 2015;112:888-93. [Crossref] [PubMed]
- Kakeji Y, Kuroda D, Nakamura T, et al. Ultrasonic shears assistance can shorten the console time in robotic gastrectomy for early gastric cancer. BMC Res Notes 2015;8:443. [Crossref] [PubMed]
- Parisi A, Ricci F, Trastulli S, et al. Robotic Total Gastrectomy With Intracorporeal Robot-Sewn Anastomosis: A Novel Approach Adopting the Double-Loop Reconstruction Method. Medicine (Baltimore) 2015;94:e1922. [Crossref] [PubMed]
- Zhang S, Jiang ZW, Wang G, et al. Robotic gastrectomy with transvaginal specimen extraction for female gastric cancer patients. World J Gastroenterol 2015;21:13332-8. [Crossref] [PubMed]
- Zhou J, Shi Y, Qian F, et al. Cumulative summation analysis of learning curve for robot-assisted gastrectomy in gastric cancer. J Surg Oncol 2015;111:760-7. [Crossref] [PubMed]
- Barchi LC, Jacob CE, Franciss MY, et al. Robotic digestive tract reconstruction after total gastrectomy for gastric cancer: a simple way to do it. Int J Med Robot 2016;12:598-603. [Crossref] [PubMed]
- Herrera-Almario G, Patane M, Sarkaria I, et al. Initial report of near-infrared fluorescence imaging as an intraoperative adjunct for lymph node harvesting during robot-assisted laparoscopic gastrectomy. J Surg Oncol 2016;113:768-70. [Crossref] [PubMed]
- Quijano Y, Vicente E, Ielpo B, et al. Full robot-assisted gastrectomy: surgical technique and preliminary experience from a single center. J Robot Surg 2016;10:297-306. [Crossref] [PubMed]
- Kim KM, An JY, Kim HI, et al. Major early complications following open, laparoscopic and robotic gastrectomy. Br J Surg 2012;99:1681-7. [Crossref] [PubMed]
- Han DS, Suh YS, Ahn HS, et al. Comparison of Surgical Outcomes of Robot-Assisted and Laparoscopy-Assisted Pylorus-Preserving Gastrectomy for Gastric Cancer: A Propensity Score Matching Analysis. Ann Surg Oncol 2015;22:2323-8. [Crossref] [PubMed]
- Park JY, Ryu KW, Reim D, et al. Robot-assisted gastrectomy for early gastric cancer: is it beneficial in viscerally obese patients compared to laparoscopic gastrectomy? World J Surg 2015;39:1789-97. [Crossref] [PubMed]
- Son T, Lee JH, Kim YM, et al. Robotic spleen-preserving total gastrectomy for gastric cancer: comparison with conventional laparoscopic procedure. Surg Endosc 2014;28:2606-15. [Crossref] [PubMed]
- Lee J, Kim YM, Woo Y, et al. Robotic distal subtotal gastrectomy with D2 lymphadenectomy for gastric cancer patients with high body mass index: comparison with conventional laparoscopic distal subtotal gastrectomy with D2 lymphadenectomy. Surg Endosc 2015;29:3251-60. [Crossref] [PubMed]
- Seo HS, Shim JH, Jeon HM, et al. Postoperative pancreatic fistula after robot distal gastrectomy. J Surg Res 2015;194:361-6. [Crossref] [PubMed]
- Okumura N, Son T, Kim YM, et al. Robotic gastrectomy for elderly gastric cancer patients: comparisons with robotic gastrectomy in younger patients and laparoscopic gastrectomy in the elderly. Gastric Cancer 2016;19:1125-34. [Crossref] [PubMed]
- Tokunaga M, Makuuchi R, Miki Y, et al. Late phase II study of robot-assisted gastrectomy with nodal dissection for clinical stage I gastric cancer. Surg Endosc 2016;30:3362-7. [Crossref] [PubMed]
- Tokunaga M, Sugisawa N, Kondo J, et al. Early phase II study of robot-assisted distal gastrectomy with nodal dissection for clinical stage IA gastric cancer. Gastric Cancer 2014;17:542-7. [Crossref] [PubMed]
- Kim HI, Han SU, Yang HK, et al. Multicenter Prospective Comparative Study of Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma. Ann Surg 2016;263:103-9. [Crossref] [PubMed]
- Park JM, Kim HI, Han SU, et al. Who may benefit from robotic gastrectomy?: A subgroup analysis of multicenter prospective comparative study data on robotic versus laparoscopic gastrectomy. Eur J Surg Oncol 2016;42:1944-9. [Crossref] [PubMed]
- Wang G, Jiang Z, Zhao J, et al. Assessing the safety and efficacy of full robotic gastrectomy with intracorporeal robot-sewn anastomosis for gastric cancer: A randomized clinical trial. J Surg Oncol 2016;113:397-404. [Crossref] [PubMed]
- Huang KH, Lan YT, Fang WL, et al. Comparison of the operative outcomes and learning curves between laparoscopic and robotic gastrectomy for gastric cancer. PLoS One 2014;9:e111499. [Crossref] [PubMed]
- Kim HI, Park MS, Song KJ, et al. Rapid and safe learning of robotic gastrectomy for gastric cancer: multidimensional analysis in a comparison with laparoscopic gastrectomy. Eur J Surg Oncol 2014;40:1346-54. [Crossref] [PubMed]
- Uyama I, Kanaya S, Ishida Y, et al. Novel integrated robotic approach for suprapancreatic D2 nodal dissection for treating gastric cancer: technique and initial experience. World J Surg 2012;36:331-7. [Crossref] [PubMed]
- Hyun MH, Park JW, Shin DS, et al. Minimizing operative time for robotic gastrectomy in cancer: analysis of the major factors for four detailed steps. Hepatogastroenterology 2014;61:2260-5. [PubMed]
- Kang BH, Xuan Y, Hur H, et al. Comparison of Surgical Outcomes between Robotic and Laparoscopic Gastrectomy for Gastric Cancer: The Learning Curve of Robotic Surgery. J Gastric Cancer 2012;12:156-63. [Crossref] [PubMed]
- Nakauchi M, Suda K, Susumu S, et al. Comparison of the long-term outcomes of robotic radical gastrectomy for gastric cancer and conventional laparoscopic approach: a single institutional retrospective cohort study. Surg Endosc 2016;30:5444-52. [Crossref] [PubMed]
- Eom BW, Yoon HM, Ryu KW, et al. Comparison of surgical performance and short-term clinical outcomes between laparoscopic and robotic surgery in distal gastric cancer. Eur J Surg Oncol 2012;38:57-63. [Crossref] [PubMed]
- Park JY, Jo MJ, Nam BH, et al. Surgical stress after robot-assisted distal gastrectomy and its economic implications. Br J Surg 2012;99:1554-61. [Crossref] [PubMed]
- Greenleaf EK, Sun SX, Hollenbeak CS, et al. Minimally invasive surgery for gastric cancer: the American experience. Gastric Cancer 2017;20:368-78. [Crossref] [PubMed]
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [Crossref] [PubMed]
- Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810-20. [Crossref] [PubMed]
- Sasako M, Sakuramoto S, Katai H, et al. Five-Year Outcomes of a Randomized Phase III Trial Comparing Adjuvant Chemotherapy With S-1 Versus Surgery Alone in Stage II or III Gastric Cancer. J Clin Oncol 2011;29:4387-93. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Tokunaga M, Tanizawa Y, Bando E, et al. Poor Survival Rate in Patients with Postoperative Intra-Abdominal Infectious Complications Following Curative Gastrectomy for Gastric Cancer. Ann Surg Oncol 2013;20:1575-83. [Crossref] [PubMed]
- Fujiya K, Tokunaga M, Mori K, et al. Long-Term Survival in Patients with Postoperative Intra-Abdominal Infectious Complications After Curative Gastrectomy for Gastric Cancer: A Propensity Score Matching Analysis. Ann Surg Oncol 2016;23:809-16. [Crossref] [PubMed]
- Kim MC, Heo GU, Jung GJ. Robotic gastrectomy for gastric cancer: surgical techniques and clinical merits. Surg Endosc 2010;24:610-5. [Crossref] [PubMed]
- Caruso S, Patriti A, Marrelli D, et al. Open vs robot-assisted laparoscopic gastric resection with D2 lymph node dissection for adenocarcinoma: a case-control study. Int J Med Robot 2011;7:452-8. [Crossref] [PubMed]
- Woo Y, Hyung WJ, Pak KH, et al. Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch Surg 2011;146:1086-92. [Crossref] [PubMed]
- Huang KH, Lan YT, Fang WL, et al. Initial experience of robotic gastrectomy and comparison with open and laparoscopic gastrectomy for gastric cancer. J Gastrointest Surg 2012;16:1303-10. [Crossref] [PubMed]
- Yoon HM, Kim YW, Lee JH, et al. Robot-assisted total gastrectomy is comparable with laparoscopically assisted total gastrectomy for early gastric cancer. Surg Endosc 2012;26:1377-81. [Crossref] [PubMed]
- Hyun MH, Lee CH, Kwon YJ, et al. Robot versus laparoscopic gastrectomy for cancer by an experienced surgeon: comparisons of surgery, complications, and surgical stress. Ann Surg Oncol 2013;20:1258-65. [Crossref] [PubMed]
- Junfeng Z, Yan S, Bo T, et al. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: comparison of surgical performance and short-term outcomes. Surg Endosc 2014;28:1779-87. [Crossref] [PubMed]
- Noshiro H, Ikeda O, Urata M. Robotically-enhanced surgical anatomy enables surgeons to perform distal gastrectomy for gastric cancer using electric cautery devices alone. Surg Endosc 2014;28:1180-7. [Crossref] [PubMed]
- Suda K, Man IM, Ishida Y, et al. Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc 2015;29:673-85. [Crossref] [PubMed]
- Cianchi F, Indennitate G, Trallori G, et al. Robotic vs laparoscopic distal gastrectomy with D2 lymphadenectomy for gastric cancer: a retrospective comparative mono-institutional study. BMC Surg 2016;16:65. [Crossref] [PubMed]
- Kim YW, Reim D, Park JY, et al. Role of robot-assisted distal gastrectomy compared to laparoscopy-assisted distal gastrectomy in suprapancreatic nodal dissection for gastric cancer. Surg Endosc 2016;30:1547-52. [Crossref] [PubMed]
- Procopiuc L, Tudor S, Manuc M, et al. Open vs robotic radical gastrectomy for locally advanced gastric cancer. Int J Med Robot 2016;12:502-8. [Crossref] [PubMed]
- Shen W, Xi H, Wei B, et al. Robotic versus laparoscopic gastrectomy for gastric cancer: comparison of short-term surgical outcomes. Surg Endosc 2016;30:574-80. [Crossref] [PubMed]
- Yang SY, Roh KH, Kim YN, et al. Surgical Outcomes After Open, Laparoscopic, and Robotic Gastrectomy for Gastric Cancer. Ann Surg Oncol 2017. [Epub ahead of print]. [PubMed]
Cite this article as: Tokunaga M, Kaito A, Sugita S, Watanabe M, Sunagawa H, Kinoshita T. Robotic gastrectomy for gastric cancer. Transl Gastroenterol Hepatol 2017;2:57.


