Three-dimensional computed tomography simulation for laparoscopic lymph node dissection in the treatment of proximal gastric cancer
Introduction
Application of laparoscopic surgery (LS) has expanded worldwide in the various fields due to the potential advantage of being less invasive than open surgery (1-3). Another advantage of LS is a capability to provide the excellent magnified views, which can be shared by a surgical team during the operation. On the other hand, disadvantages of LS may be loss of tactile senses as well as subsequent difficulty in confirming positional relationships of anatomical structures, which can lead to bleeding or organ injuries during the operations. In order to compensate these disadvantages of LS, image navigation system is worth evaluating. Image navigation system for surgery is developing year by year thanks to the progress of imaging technologies and could be classified as two subtypes: one is “navigation”, in a narrow sense, which guides surgical procedures in real-time using the imposed images, and the other is simply “simulation” done before surgery, which can be referred by surgeons during the operation. Generally, this “simulation” images are created using data obtained by multi detector-row computed tomography (MDCT). These data can be processed to three-dimensional (3D) anatomical reconstruction by the specified image-analyzing software. Preoperative anatomical reconstruction for solid organs, such as the liver or the kidney, has already been widely utilized in clinical practice (4,5), however for the digestive tract, there is a big problem of influences from the respiratory or physiologically movement, resulting in discrepant images. To solve this problem, the latest software has an adjustment-function, or setting of suitable timing of CT scanning enables proper delineation.
It is known that there is a remarkable anatomical variation in the area of the splenic hilum or of the splenic vessels’ branching. Furthermore, complicated positional relationships make the lymph node dissection of this region extremely difficult, though which has an oncological impact to proximal advanced gastric cancer (6). In addition, inadequate surgical manipulation around this region may cause intraoperative hemorrhage, splenic infarction or postoperative pancreatic fistula. In this context, 3D-CT anatomical simulation is thought to be very effective for this procedure, and we have routinely employed it when performing the splenic hilar lymph node dissection.
There have been several publications reporting effectiveness of 3D-CT anatomical simulation in gastric cancer surgery, however most of which was evaluating the anatomical diversity of the celiac axis (7-10). As for 3D-CT anatomical simulation about surgical anatomy around the splenic hilum, only few articles have been published so far (11-14). In this article, we introduce the method of 3D-CT anatomical simulation performed in our institution, and additionally review the previous publications regarding this issue.
Indications of the splenic hilar dissection
Japan Clinical Oncology Group (JCOG) conducted a randomized controlled trial, comparing splenectomy and non-splenectomy in the treatment of proximal advanced gastric cancer without the greater curvature invasion (JCOG0110). Long-term outcomes of this study have shown no survival benefit of splenectomy, but resulted in enhancing morbidity rate (15). Previous articles reported that morbidity rate of total gastrectomy with splenectomy, either by open surgery or LS, as 9.6–33.3% (16,17). From the immunologic viewpoints, splenectomy may be associated with loss of immune function. Therefore, unless cT3/T4 tumors invade the greater curvature or directly involve the splenogastric ligament, splenectomy should be avoided. Spleen-preserving splenic hilar dissection is thought to be an alternative for avoiding splenectomy. However, skeletonizing vascular structures at the splenic hilum is technically demanding even for well-experienced surgeons. Following the insights obtained from JCOG 0110, for tumors not invading the greater curvature line, complete removal of No. 10 nodal station is not necessary.
Our protocol for 3D-CT anatomical reconstruction
As we reported, in our institution, CT scanning is performed using a 64-row MDCT scanner (Aquilion 64TM; Toshiba Medical Systems, Tochigi, Japan) with 0.5 mm slice thickness (12). Intravenous injection of a nonionic soluble contrast medium was performed at a rate of 3.0 mL/s to a total volume of 100 mL. Images were obtained during the arterial phase with bolus tracking technique and during the portal phase at 80 s after the start of injection. Using Digital Imaging and Communication in Medicine (DICOM) data, 3D images were created with the use of volume analyzing software, synapse VINSENTTM (Fujifilm Medical, Tokyo, Japan).
Anatomical images of the artery, the vein, the pancreas and the spleen were created individually. In most of cases, all subjects were reconstructed using only arterial phase data, and then fused to create 3D images. In some cases, low enhancement of the splenic vein is recognized at the arterial phase. For such cases, images gained at the portal phase are used to be fused later.
Case example 1 (Figure 1A,B,C)
A 48-year-old male patient with type 2 tumor located at the posterior wall of the upper stomach underwent laparoscopic total gastrectomy with spleen-preserving splenic hilar dissection. Before surgery, 3D-CT anatomical simulation was employed. This simulation gave us helpful information in advance as following: (I) the main trunk of the splenic artery is mostly apart from the splenic vein; (II) the upper pole branch derives from the proximal site of the splenic artery; (III) the posterior gastric artery derives from the upper pole branch. In this simulation image, several short gastric vessels were also detectable. When we perform laparoscopic spleen-preserving splenic hilar dissection, the operation table is in head-up and left side up tilt position. First, the pedicle of the left gastroepiploic vessel is retracted, and the caudal aspect of the splenic hilum is identified. The left gastroepiploic vessel is divided at its origin from the inferior branch of the splenic artery and vein (12). Then, the main trunk of the splenic artery before its bifurcation is identified, and the dissection is continued from proximal to distal, towards the splenic hilum. The branches of the splenic artery and vein are skeletonized until reaching the splenic parenchyma to remove No. 11d and No. 10 station lymph nodes, dividing several short gastric vessels at their roots. Finally, lymph node dissection around the superior branch or the upper pole branch is accomplished.
Case example 2 (Figure 1D,E,F)
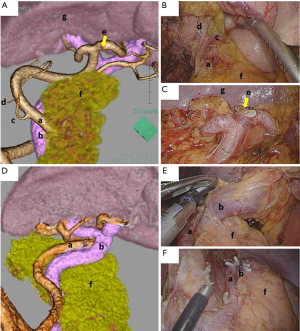
A 31-year-old female with type 4 advanced gastric cancer, involving the entire circumference of the stomach, underwent laparoscopic total gastrectomy with splenectomy. Before surgery, 3D-CT simulation was employed, which showed moderately meandering splenic artery and vein. The upper pole branch was not detected. As for positional relationship at the splenic hilum, the vein is mainly located anterior to the artery. In performing splenectomy, the distance between the pancreatic tail and the spleen is quite important, which may indicate the difficulty of this procedure. Namely, if the distance is relative long, more than 2 cm, the splenic artery or vein would be divided at their trunk, but if the distance is short or almost adjacent, the splenic artery or vein would be divided after complex bifurcations, which may dramatically increase surgical difficulty. In addition, in some cases, the caudal pancreatic artery or vein can be detected by 3D-CT simulation, which preservation is thought to be crucial to prevent postoperative pancreatic fistula.
Previous publications regarding 3D-CT simulation for gastric cancer surgery
Miyamoto et al. reported the usefulness of 3D-CT image to understand the anatomy of the common hepatic artery and the left gastric vein in LS. They reported a significant reduction of intraoperative blood loss by using 3D-CT simulation (8). Similarly, Natsume et al. investigated the branch patterns of the celiac axis and the left gastric vein (18). They emphasized that 3D-CT is an essential tool for laparoscopic gastric cancer surgery. In terms of the splenic hilar dissection, Wang et al. reported usefulness of 3D-CT for spleen-preserving splenic hilar dissection (13), with their consequent series of 312 cases. Authors compared the surgical outcomes of surgeries with or without preoperative 3D-CT simulation, and they revealed that operation time and blood loss could be significantly reduced by using 3D-CT simulation. Furthermore, Hyung et al. and Kinoshita et al. reported their procedures of laparoscopic spleen-preserving splenic hilar dissection with 3D-CT simulation (11,12). Kinoshita et al. reported that there was no statistical difference in operation time, blood loss or complication rate, however, number of retrieved No. 10 LN was significantly larger in cases with the use of 3D-CT, suggesting that 3D-CT might enhance the surgical quality by more precise and accurate dissection.
Conclusions
According to the published data or our experiences, 3D-CT anatomical simulation seems to be effective for the splenic hilar lymph node dissection to reduce technical difficulty as well as increase surgical quality.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kitano S, Shiraishi N, Fujii K, et al. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery 2002;131:S306-11. [Crossref] [PubMed]
- Zeng YK, Yang ZL, Peng JS, et al. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: evidence from randomized and nonrandomized clinical trials. Ann Surg 2012;256:39-52. [Crossref] [PubMed]
- Sakuramoto S, Yamashita K, Kikuchi S, et al. Laparoscopy versus open distal gastrectomy by expert surgeons for early gastric cancer in Japanese patients: short-term clinical outcomes of a randomized clinical trial. Surg Endosc 2013;27:1695-705. [Crossref] [PubMed]
- Kamiyama T, Nakagawa T, Nakanishi K, et al. Preoperative evaluation of hepatic vasculature by three-dimensional computed tomography in patients undergoing hepatectomy. World J Surg 2006;30:400-9. [Crossref] [PubMed]
- Komai Y, Sakai Y, Gotohda N, et al. A novel 3-dimensional image analysis system for case-specific kidney anatomy and surgical simulation to facilitate clampless partial nephrectomy. Urology 2014;83:500-6. [Crossref] [PubMed]
- Watanabe M, Kinoshita T, Enomoto N, et al. Clinical Significance of Splenic Hilar Dissection with Splenectomy in Advanced Proximal Gastric Cancer: An Analysis at a Single Institution in Japan. World J Surg 2016;40:1165-71. [Crossref] [PubMed]
- Huang Y, Mu GC, Qin XG, et al. Study of celiac artery variations and related surgical techniques in gastric cancer. World J Gastroenterol 2015;21:6944-51. [PubMed]
- Miyamoto R, Inagawa S, Nagai K, et al. Three-dimensional reconstruction of vascular arrangement including the hepatic artery and left gastric vein during gastric surgery. Springerplus 2016;5:835. [Crossref] [PubMed]
- Mu GC, Huang Y, Liu ZM, et al. Clinical research in individual information of celiac artery CT imaging and gastric cancer surgery. Clin Transl Oncol 2013;15:774-9. [Crossref] [PubMed]
- Yamashita K, Sakuramoto S, Mieno H, et al. Preoperative dual-phase 3D CT angiography assessment of the right hepatic artery before gastrectomy. Surg Today 2014;44:1912-9. [Crossref] [PubMed]
- Hyung WJ, Lim JS, Song J, et al. Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg 2008;207:e6-11. [Crossref] [PubMed]
- Kinoshita T, Shibasaki H, Enomoto N, et al. Laparoscopic splenic hilar lymph node dissection for proximal gastric cancer using integrated three-dimensional anatomic simulation software. Surg Endosc 2016;30:2613-9. [Crossref] [PubMed]
- Wang JB, Huang CM, Zheng CH, et al. Role of 3DCT in laparoscopic total gastrectomy with spleen-preserving splenic lymph node dissection. World J Gastroenterol 2014;20:4797-805. [Crossref] [PubMed]
- Zheng CH, Xu M, Huang CM, et al. Anatomy and influence of the splenic artery in laparoscopic spleen-preserving splenic lymphadenectomy. World J Gastroenterol 2015;21:8389-97. [Crossref] [PubMed]
- Sano T, Sasako M, Mizusawa J, et al. Randomized Controlled Trial to Evaluate Splenectomy in Total Gastrectomy for Proximal Gastric Carcinoma. Ann Surg 2017;265:277-83. [Crossref] [PubMed]
- Lee JH, Ahn SH, Park DJ, et al. Laparoscopic total gastrectomy with D2 lymphadenectomy for advanced gastric cancer. World J Surg 2012;36:2394-9. [Crossref] [PubMed]
- Nakata K, Nagai E, Ohuchida K, et al. Technical feasibility of laparoscopic total gastrectomy with splenectomy for gastric cancer: clinical short-term and long-term outcomes. Surg Endosc 2015;29:1817-22. [Crossref] [PubMed]
- Natsume T, Shuto K, Yanagawa N, et al. The classification of anatomic variations in the perigastric vessels by dual-phase CT to reduce intraoperative bleeding during laparoscopic gastrectomy. Surg Endosc 2011;25:1420-4. [Crossref] [PubMed]
Cite this article as: Sunagawa H, Kinoshita T. Three-dimensional computed tomography simulation for laparoscopic lymph node dissection in the treatment of proximal gastric cancer. Transl Gastroenterol Hepatol 2017;2:54.


