Percutaneous endoscopic intragastric surgery: an organ preserving approach to submucosal tumors at esophagogastric junction
Introduction
The goal of surgical treatment for primary submucosal tumor of the stomach is R0 resection (1-3). Because such lymph node dissection as in surgery for gastric cancer is not necessary for most of gastric submucosal tumors, it should be preferable to select such a resection method as can preserve both of the configuration and function of the stomach to maintain the quality of life of patient. For submucosal tumors located distant from the esophagogastric junction (EGJ), it is not necessarily difficult to perform wedge resection with preserving the stomach as much as possible. However, for those located in EGJ, total or proximal gastrectomy is often selected due to its anatomical difficulty.
In order to preserve the entire stomach of the patients with a tumor at EGJ the authors have been performing percutaneous endoscopic intragastric surgery (PEIGS) since 1993 and have reported its fair outcomes (4-7). Moreover, in addition to the original method of PEIGS, we have recently developed further cosmetic procedures such as single incision PEIGS (5,6) and needlescopic PEIGS (7). Herein we demonstrate the techniques of the three different types of PEIGS, which should play an important role as an organ preserving surgery in the treatment of gastric submucosal tumors.
Patient selection
When a submucosal tumor located at EGJ is approached from outside the stomach and an ideal resection with necessary safety margin is attempted, it results in complete separation of distal esophagus and the cardia. Even when complete separation is avoided, problems such as division of the vagal nerve trunk, breaking the anti-reflux mechanism, and/or stenosis in the passage, would lead to a significant morbidity. Due to these anatomical difficulties, major gastrectomy such as total or proximal gastrectomy is usually chosen to resect the tumor at EGJ. PEIGS is best indicated for such cases to salvage the stomach.
We strictly limit the indication of PEIGS only for the tumors at EGJ (at 0 to 2 cm from EGJ). Lesions on the anterior wall, as far as it is located at exact EGJ, are also good candidates for PEIGS. When the tumor has significant exophytic growth, significant extension to the esophagus, or originating from the esophagus, PEIGS is not indicated. For such tumors we approach by laparoscopy in the peritoneal cavity to perform enucleation with leaving the mucosal layer unbroken, followed by fundoplication (8).
Tumor size limitation is a controversial issue as to the indication of PEIGS. In most guide-lines laparoscopic or endoscopic surgical treatment is not recommended for the tumors measuring 5 cm or larger (9-11). But recent literatures suggest that one is not necessarily discouraged to perform laparoscopic surgery for submucosal tumors >5 cm (12-14). Our previous publication also demonstrated convincing clinical results from PEIGS for large tumors (max. 8 cm), and thus suggested submucosal tumors >5 cm can be treated by PEIGS, when it is carefully performed by experienced laparoendoscopic surgeon (6).
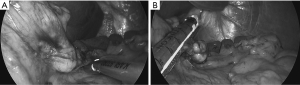
When the tumor is apart from EGJ (>2 cm from EGJ), another option such as wedge resection from serosal aspect of the stomach shall be indicated. Even when the tumor is located on the posterior wall, if there is a distance >2 cm from EGJ, it can be treated by laparoscopic full-thickness partial gastrectomy. For this procedure the omentum is opened and the stomach is flipped over to expose the posterior wall of the cardia (Figure 1A). Even when the tumor is endophytic in this particular location and simple stapling wedge resection seems unfavorable due to the risk of stenosis, CLEAN-NET (15) can solve this problem and achieve complete full-thickness resection without significant deformity in EGJ (Figure 1B).
Operative techniques
Original PEIGS
Setup: the patient is placed supine with legs spread apart. The surgeon stands between the patient’s legs, and the assistant surgeon stands on the right side of the patient to hold the laparoscope. The scrub nurse stands on the left side of the patient.
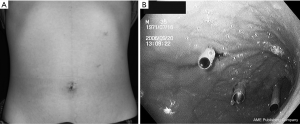
Establishment of the access route: for the first trocar, the navel is incised 2 cm longitudinally, through which the abdominal cavity is entered. When the stomach does not reach the navel, the site for first skin incision is shifted in the cephalic direction between the navel and the xhyphoid process. Through the first incision a 12 mm port is inserted and pneumoperitoneum is started. Then the peritoneal cavity is scanned to check up if there is not adhesion around the stomach. Pneumoperitoneum is then stopped and the port is removed. Intraoperative upper GI endoscopy is carried out to inflate the stomach so that the stomach is stretched to reach the first skin incision site. The anterior wall of the stomach is percutaneously fixed to the abdominal wall on each lateral side to the first skin incision area by suture with Funada’s gastropexy instrument (Clinit Medical Co. Ltd, Osaka, Japan) (4). With this fixation the following insertion of the intragastric trocar becomes stable. Through the first incision site a 12 mm expandable port (STEP Trocar®, Covedien, USA) is inserted into the gastric lumen. In addition, two 5 mm ports are placed in the left upper quadrant, penetrating into the anterior wall of the middle to upper gastric body. Again 5 mm STEP Trcars® are used for these two sites (Figure 2A,B). The gastric lumen is insufflated with CO2 gas at 8 to 10 mmHg. A 30 degree oblique view laparoscope (5 mm) is used via the middle port to visualize EGJ and the lesion.
Intragastric procedure: an injection needle catheter (Pettit Needle®, Hakko Company, Nagano, Japan) is inserted through an intragastric port. Normal saline is injected into the submucosa around the tumor. The mucosa around the tumor is cut with an electrocautery hook (Figure 3A). To facilitate the mucosa is retracted by a grasping forceps inserted in the other port. The submucosa is consequently exposed and dissected until the tumor surface (pseudocapsule) is identified through submucosa which became transparent with saline. The muscle layer around the tumor is cut with attention avoiding the damage onto the pseudocapsule (Figure 3B). When tumor is extirpated, in the bottom of the defect, the peri-gastric fat tissue is often seen (Figure 3C). The specimen is entrapped in a retrieval bag, which is finally brought out of the mouth by flexible endoscope (Figure 3D). The defect on EGJ wall is closed by hand-sew/interrupted sutures with 3-0 absorbable thread (Figure 3E).
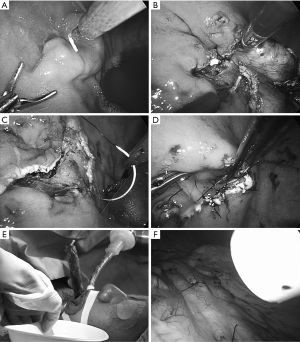
Intra-abdominal procedure: the fixation thread at the port-sites are cut and released. Then all three intragastric ports are pulled out the gastric wall but kept staying in the peritoneal cavity. The three stab wounds on the gastric wall are closed by hand-sew (Figure 3F).
Single incision PEIGS
Setup: the same manner is applied as in “Original PEIGS”.
Construction of a temporary gastrostomy: The navel is incised 2.5 cm longitudinally to enter the peritoneal cavity. When the stomach does not touch the navel, the incision site is shifted to cephalad accordingly. Through this incision, the stomach is pulled out and a gastrostomy is constructed. The inner ring of the x-Gate® (16) (a multichannel port developed for single incision endoscopic surgery; Sumitomo Bake-Lite Japan) is inserted into the stomach via the gastrostomy. Then the working insert of the x-Gate® is attached to the outer ring. The stomach is insufflated with carbon dioxide gas at 8 mmHg. A needle port (BJ port®, Nition Company, Chiba, Japan) is punctured percutaneously and inserted into the stomach in the left subcostal margin, through which a variety of 2 mm instruments (electrode, grasper, scissors, etc.) (17) are brought in (Figure 4A,B,C).

Intragastric procedure: A rigid 5 mm endoscope, and a 5 mm instrument are inserted into the stomach via the channels of x-Gate®, while a 2mm instrument is used through the BJ port®. The intragastric procedure is almost the same manner as described in “original PEIGS” (Figure 5A,B,C,D). The specimen is entrapped in a retrieval bag, which is then extracted through the x-Gate® (Figure 5E).
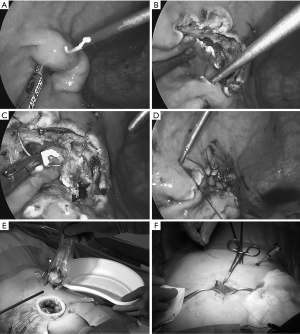
Repair of the gastrostomy: The main body of x-Gate® is removed and the gastrostomy is closed by suturing extracorporeally (Figure 5F). The tiny puncture wound in the upper gastric body made by the BJ port® is left untreated.
Needlescopic PEIGS
Needlescopic surgical instruments (2 mm instruments).
We developed a series of 2 mm instruments in corporation with Niti-On Co., Ltd. (Chiba, Japan). These instruments are called BJ series including grasping forceps (BJ needle®), metal trocar and cannula (BJ port®), rigid endoscope with 30 degree oblique view (BJ scope®), monopolar electrocautery with a hook-shaped electrode (BJ hook®), scissors (BJ scissors®), and needle driver (BJ pico®) (17). These instruments are autoclavable.
Setup: the same manner is applied as in “Original PEIGS”.
Establishment of the access ports: the first puncture site is determined by the same technique described in the previous operative techniques. When the stomach stretches to the navel, navel is incised 5 mm longitudinally, while the navel is not reached by the stomach, the first puncture site must be shifted to cephalad in an appropriate part of the epigastrium, where the stomach can reach. Fixation of the gastric wall and the abdominal wall is performed by the same manner described in the previous procedures. Under the control of peroral endoscopy, a 5 mm STEP® trocar is inserted rough this incision into the gastric lumen. Then the stomach is dilated by insufflation with CO2 gas. Two 2 mm ports are punctured and inserted into the gastric lumen in the left subcostal area (Figure 6A,B).
Intragastric procedure: with a 5mm laparoscope, inserted via the 5mm port, the inside of the gastric lumen is visualized. Resection is performed mainly with two 2 mm instruments (BJ hook® and BJ needle®) (Figure 7A, B). The defect in the EGJ is closed by an interrupted suture with a 4/0 absorbable monofilament thread with a 17 mm 3/8 circle needle. Hand-sew in this operation is facilitated by usage of BJ pico®, a 2 mm needle holder (Figure 7C). The specimen, is entrapped in a retrieval bag, which is home-made by cutting the thumb of the surgical-glove and applying purse-string suture along its rim to pass the 5 mm port (Figure 7D). The bag is extracted via the esophageal-oral route as described before.
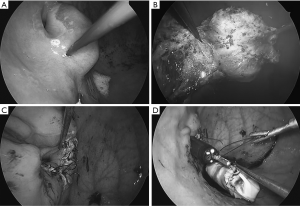
Intra-abdominal procedure: after all procedures are carried out in the stomach, the three intragastric ports are withdrawn from the stomach. They are kept remaining inside the abdomen. The 5 mm stab-wound on the gastric wall is sutured and closed with 4-0 absorbable thread. The pinholes (2 mm puncture sites) on the gastric wall are left untreated.
Postoperative care
Patients receive intravenous infusion, antibiotics, and proton-pump inhibitor on the day of surgery through POD2. Proton-pump inhibitor administration is maintained as tablets for 6 weeks thereafter. Patients start to drink clear fluid on POD1, eat soup and puree on POD3, and porridge or similar soft diet on POD4. Patients are discharged from the hospital on POD6 or 7 in case the course is uneventful.
Confusing terminology
In 1993 Ohashi (18) developed a unique operative procedure and named it intragastric surgery, by which a tumorous lesion is resected inside the stomach by using a telescope and two surgical instruments brought in through the three ports, which are penetrating the abdominal wall and the anterior gastric wall. A couple of months later Ohgami (19), and Kanehira (4) also started this operation and reported its ability to perform precise excision of early gastric cancer. Since then some reports on the same or similar surgical procedures have been published (20-28), although the major spread of this type of operation has not been seen. In the past literatures several different terminologies were used such as intragastric surgery (20-22), transgastric surgery (23-25), endoluminal gastric surgery (26-28), etc. The authors are convinced we should to appreciate the priority of the first surgeon who developed this operation and named it, and propose the original terminology, “intragastric surgery” (18). Besides, some use “laparoscopic”, while the other use “endoscopic”, for this operation, which may lead to another confusion. We strongly insist on “endoscopic”. Firstly because, when a telescope is once brought into the stomach, it is not a laparoscope any longer, but shall be called “gastroscope”. But the procedures we describe herein include also intraperitoneal part, during which the telescope should be called “laparoscope”. In this context, to indicate the entire operation, it is not only gastroscopic, neither only laparoscopic, but must be called “endoscopic” surgery. Moreover as there are two routes to reach inside the stomach endoscopically, namely peroral and percutaneous, we should clarify it and add “percutaneous”. These are the reasons why we recommend the terminology “percutaneous endoscopic intragastric surgery”.
Technical tips
Establishment of the stable access routes is the most important key to make this operation successful. At the beginning we used the port with a balloon (4). The idea was to avoid from slipping withdrawal of the port. But we experienced burst of the balloon by hitting with a surgical instrument during the operation many times. Then the balloon ports are replaced by the ports with an expandable sheath (STEP-trocar®), which we find the most ideal one for the stability in access routes. In addition, the fixation of the gastric wall and the abdominal wall improves the stability in the access port significantly. We apply this in bilateral area at each port. When the trocar is punctured those fixation enable to create appropriate resistance to help the safe puncture. Moreover when the port is accidentally withdrawn during the operation the fixation keeps the location of the port-sites both on the abdominal wall and gastric wall, which facilitates safe re-insertion of the port through the previous route. We believe that our good operative outcomes are attributed to our way of establishing the access routes.
Developing a technique of single incision PEIGS was a challenge for us. But once it was put into a clinical practice the learning curve was very quick and settled within 20 cases. Over the last 4 years [2013–2016] about 70% of our series of PEIGS have been performed by single incision approach. The outstanding technical difficulty of this operation is to use the surgeon’s left hand for the main instruments such as an energy device or a needle holder. Right-handed surgeons must be well trained to use their left hand for single incision PEIGS. The advantage is to enable the extraction of a larger tumor. In conventional PEIGS or needlescopic PEIGS the specimen extraction is carried out by NOSE (natural orifice specimen extraction) via esophago-oral route. This technique limits the specimen size up to 3 to 4 cm in diameter. However, in single incision PEIGS, the diameter of gastrostomy can be extended according to the tumor size, so that the specimen can be extracted safely via the gastrostomy.
Needlescopic PEIGS (7) maybe the highest end of PEIGS series. This minimal access surgery has a lot of requirements. It is not common to be equipped with a variety of 2 mm instruments. Ability to find out and coagulate a potential bleeder before it is cut is important as once bleeding occurs it is not easily controlled by 2 mm instruments. Manipulating the thinnest caliber (2 mm) instruments requires meticulous handling, especially in hand-sew. It is not easy to drive precisely a small size needle and approximate the esophageal and gastric walls. However, if needlescopic PEIGS is performed successfully it can preserve the stomach, and also minimize the invasion to the abdominal wall. Of course, its cosmetic results are satisfactory, remaining only 5, 2, and 2 mm scars.
A modification
There have been reports on similar procedures with modification. Tumor resection with endosurgical stapler performed inside the stomach could be an option, which reduces the operation time significantly (23,24). But the indication of such a modification should be limited only when the target is apart from the EGJ, because when the tumor is exactly at EGJ application of endosurgical stapler should be difficult. When distance between the tumor and EGJ is 2 cm or longer, full thickness wedge resection from the peritoneal cavity can also be applied. It means such tumors as can be resected by stapling PEIGS, can also be resected by wedge resection from outside the stomach. Moreover, one of the risks in stapling PEIGS is that when the stapler is used inside the stomach, adjacent tissue can be also clamped and eventually cut. If the short gastric vessels, for example, are clamped and cut and if they bleed, it cannot be seen and controlled from inside. Those are the reasons why we think the indication of stapling PEIGS should be carefully considered. And we suggest not select this modification only because the surgeon does not like to perform hand-sew.
Short-term outcomes
There have been very few clinical reports dealing purely with PEIGS with a large number of patients. Most of them were case reports, or the mixed reports with patients undergoing other procedures such as laparoscopic wedge resection, laparoscopic endoscopic cooperative surgery, etc. However, Kanehira et al. (6) (the current authors included) have reported on the outcomes of pure PEIGS from relatively large number of patients (n=59). According to their reports the operation is performed with very low morbidity rate. The outcomes regarding the completeness of resection were also excellent. R0 resection was achieved in all patients undergoing PEIGS. However they cautioned that PEIGS must be performed by experienced surgeons especially with an enough skill of hand-sew in narrow space.
Oncological outcomes
For an oncological assessment of long-term outcomes from surgery for submucosal tumors of the stomach, GIST must be picked up. There have been only few literatures with long-term outcomes of patients with gastric GIST treated by PEIGS. Moreover, it is often difficult to extract the purified data of only PEIGS cases, while they contain mixed operative methods and results of unclassified groups. Mino et al. (29) reported the long-term outcomes of 15 cases, who were followed up for max. 61 months and presented no recurrence. The report by Novitsky et al. (30) included a variety of operative methods. Twenty patients of their series seem to have undergone PEIGS, who, they reported, survived up to 84 months without local recurrence. The report by Kanehira et al. (6) (including current authors) has the largest number of patients with long-term outcomes. All of 59 patients in their series undergoing PEIGS for GIST survived up to 108 months without local recurrence. Although in Novitsky’s report and Kanehira’s report there were patients with recurrence in the form of liver metastasis, it is not supposed to be attributed to the operative technique, but should be due to original nature of the tumor.
Conclusions
PEIGS has not been commonly performed up to now, and there have been only few literatures with only small number of cases on this unique operation. Since 1993 the author has been performing PEIGS in more than 150 cases and reported good early- and long-term outcomes in GIST cases. Also we have developed single incision PEIGS and needlescopic PEIGS, both of which showed also good results. By reviewing past literatures and our own results, PEIGS can be performed in hands of experienced endoscopic surgeons and it should play a most important role to salvage the stomach in patients with submucosal tumors at EGJ.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Everett M, Gutman H. Surgical management of gastrointestinal stromal tumors:analysis of outcome with respect to surgical margins and technique. J Surg Oncol 2008;98:588-93. [Crossref] [PubMed]
- Scherübl H, Faiss S, Knoefel WT, et al. Management of early asymptomatic gastrointestinal stromal tumors of the stomach. World J Gastrointest Endosc 2014;6:266-71. [Crossref] [PubMed]
- Koo DH, Ryu MH, Kim KM, et al. Asian Consensus Guidelines for the Diagnosis and Management of Gastrointestinal Stromal Tumor. Cancer Res Treat 2016;48:1155-66. [Crossref] [PubMed]
- Kanehira E, Omura K, Wakasa E, et al. A technique of percutaneous endoscopic intragastric surgery. Minim Invasive Ther Allied Technol 1998;7:15-20. [Crossref]
- Kanehira E, Kamei A, Tanida T. Wedge gastric and Endo-gastric resection. In: Mori T, Dapri G. editors. Reduced Port Laparoscopic Surgery, Sprnger, Tokyo 2014:221-31.
- Kanehira E, Kamei A, Umezawa A, et al. Long-term outcomes of percutaneous endoscopic intragastric surgery in the treatment of gastrointestinal stromal tumors at the esophagogastric junction. Surg Endosc 2016;30:2036-42. [Crossref] [PubMed]
- Kanehira E, Tanida T, Kamei A, et al. Needlescopic intragastric surgery facilitated by newly developed 2mm instruments. Minim Invasive Ther Allied Technol 2016;25:210-4. [Crossref] [PubMed]
- Kanehira E, Tanida T, Kamei A, et al. Minimally Invasive, Organ-preserving Surgery for Large Submucosal Tumors in the Abdominal Esophagus. Surg Laparosc Endosc Percutan Tech 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Blay JY, Bonvalot S, Casali P, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol 2005;16:566-78. [Crossref] [PubMed]
- Demetri GD, Benjamin RS, Blanke CD, et al. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw 2007;5 Suppl 2:S1-29; quiz S30.
- Nishida T, Hirota S, Yanagisawa A, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan:English version. Int J Clin Oncol 2008;13:416-30. [Crossref] [PubMed]
- Goh BK, Chow PK, Chok AY, et al. Impact of the introduction of laparoscopic wedge resection as a surgical option for suspected small/medium-sized gastrointestinal stromal tumors of the stomach on perioperative and oncologic outcomes. World J Surg 2010;34:1847-52. [Crossref] [PubMed]
- Karakousis GC, Singer S, Zheng J, et al. Laparoscopic versus open gastric resections for primary gastrointestinal stromal tumors (GISTs):a size-matched comparison. Ann Surg Oncol 2011;18:1599-605. [Crossref] [PubMed]
- Koh YX, Chok AY, Zheng HL, et al. A systematic review and meta-analysis comparing laparoscopic versus open gastric resections for gastrointestinal stromal tumors of the stomach. Ann Surg Oncol 2013;20:3549-60. [Crossref] [PubMed]
- Inoue H, Ikeda H, Hosoya T, et al. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond:full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET). Surg Oncol Clin N Am 2012;21:129-40. [Crossref] [PubMed]
- Kanehira E, Siozawa K, Kamei A, et al. Development of a novel multichannel port (x-Gate(®)) for reduced port surgery and its initial clinical results. Minim Invasive Ther Allied Technol 2012;21:26-30. [Crossref] [PubMed]
- Kamei A, Kanehira E, Nakagi M, et al. Development of scar-less laparoscopic hernia repair (TAPP-252) facilitated by new 2mm instruments. Minim Invasive Ther Allied Technol 2016;25:314-8. [Crossref] [PubMed]
- Ohashi S. Laparoscopic intraluminal (intragastric) surgery for early gastric cancer. A new concept in laparoscopic surgery. Surg Endosc 1995;9:169-71. [Crossref] [PubMed]
- Ohgami M, Otani Y, Kumai K, et al. Curative laparoscopic surgery for early gastric cancer:five years experience. World J Surg 1999;23:187-92. [Crossref] [PubMed]
- Walsh RM, Ponsky J, Brody F, et al. Combined endoscopic/laparoscopic intragastric resection of gastric stromal tumors. J Gastrointest Surg 2003;7:386-92. [Crossref] [PubMed]
- Wong DC, Wong SK, Leung AL, et al. Combined endolaparoscopic intragastric excision for gastric neoplasms. J Laparoendosc Adv Surg Tech A 2009;19:765-70. [Crossref] [PubMed]
- Boulanger-Gobeil C, Gagné JP, Julien F, et al. Laparoscopic Intragastric Resection:An Alternative Technique for Minimally Invasive Treatment of Gastric Submucosal Tumors. Ann Surg 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Barajas-Gamboa JS, Acosta G, Savides TJ, et al. Laparo-endoscopic transgastric resection of gastric submucosal tumors. Surg Endosc 2015;29:2149-57. [Crossref] [PubMed]
- Xu X, Chen K, Zhou W, et al. Laparoscopic transgastric resection of gastric submucosal tumors located near the esophagogastric junction. J Gastrointest Surg 2013;17:1570-5. [Crossref] [PubMed]
- Hwang SH, Park DJ, Kim YH, et al. Laparoscopic surgery for submucosal tumors located at the esophagogastric junction and the prepylorus. Surg Endosc 2009;23:1980-7. [Crossref] [PubMed]
- Lai DT, Chu KM, Storey DW. Laparoscopic endoluminal gastric surgery. Aust N Z J Surg 1996;66:41-2. [Crossref] [PubMed]
- Spivak H, Hunter JG. Endoluminal surgery. Surg Endosc 1997;11:321-5. [Crossref] [PubMed]
- Rosen MJ, Heniford BT. Endoluminal gastric surgery: the modern era of minimally invasive surgery. Surg Clin North Am 2005;85:989-1007. [Crossref] [PubMed]
- Mino JS, Guerron AD, Monteiro R, et al. Long-term outcomes of combined endoscopic/laparoscopic intragastric enucleation of presumed gastric stromal tumors. Surg Endosc 2016;30:1747-53. [Crossref] [PubMed]
- Novitsky YW, Kercher KW, Sing RF, et al. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg 2006;243:738-45. [Crossref] [PubMed]
Cite this article as: Kanehira E, Kanehira AK, Tanida T, Takahashi K, Sasaki K. Percutaneous endoscopic intragastric surgery: an organ preserving approach to submucosal tumors at esophagogastric junction. Transl Gastroenterol Hepatol 2017;2:48.


