Current practice for gastric cancer treatment in Ukraine
Introduction
Gastric cancer (GC) is a significant and unsolved problem, despite a slight decrease in its morbidity. Globally, 952,000 new GC cases were registered in 2012. In South Korea and Japan, because of screening for GC, the 5-year survival rate is more than 60%. In most other countries, this value is 2–3 times lower (1,2). In Ukraine, GC is a serious problem. Annually, the country registers more than 8,000 new GC cases. The disease is usually diagnosed at stage III–IV (65% of cases) and 62.2% of patients die within 1 year. About 70% of patients with GC need chemotherapy, and for most of them, this is the only way to increase their life expectancy.
Morbidity and death rate
According to the national cancer register of Ukraine, there were 8,350 new cases of GC in 2014 (5,104 in men and 3,246 in women). Thus, the morbidity rate is 23.0 cases per 100,000 people (30.3 for men and 16.7 for women), which puts the disease in fourth place for men and eighth place for women for morbidity as a result of malignant tumors in Ukraine. During the period under investigation, 6,414 patients diagnosed with GC died. The death rate was 17.7 cases per 100,000 people (23.8 for men and 12.3 for women), which means that, for men and women respectively, GC has the second and third highest death rates from malignant tumors in Ukraine.
People diagnosed with GC for the first time are diagnosed at the following stages according to the TNM classification: stage I–II, 37.6%; stage III, 22.5%; stage IV, 33.9%; and indistinct stage, 5%. Thus, advanced GC is diagnosed for the first time in as many as 60% of all patients, which leads to a high death rate of more than 50% during the first year.
The above information allows us to conclude that the early diagnosis of GC, as well as combined methods of treating it with neo-adjuvant and adjuvant therapy, is of paramount importance in Ukraine.
Despite the gradual decline in GC morbidity, there are still some serious associated problems. Namely, in the last 10 years, the following was noted (Table 1): (I) very few changes in the morbidity rate for women; (II) decrease in the diagnosis of stage I–II disease in spite of the necessary endoscopic equipment; (III) high death rate within 1 year.
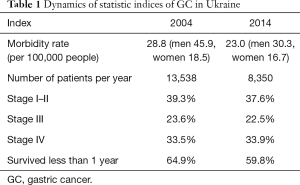
Full table
Given this information, we should define the most important aspects of the diagnosis and treatment of GC in Ukraine, which would help improve the situation and achieve better results.
Diagnosis
Ukraine still has a relatively inefficient method of diagnosing GC, which is based on “appealability”. It accounts for 70% of cases in which an advanced tumor is diagnosed, which explains why the 5-year survival rate for GC patients does not exceed 13.8%.
It is noteworthy that, because of the high cost and complexity, GC screening is only carried out in some countries of South East Asia that have high GC morbidity rates. Contrast fluorography and fibrogastroscopy (FGS) are the most efficient methods of GC screening. FGS is three times less expensive for early diagnosis, although it does require greater skills from the endoscopist (3,4).
It is but natural that it is the real situation in the country that should underlie the program aimed at solving the problem of GC.
Regular FGS for patients with pre-cancerous pathology and a high risk of developing GC could be the most affordable method of early diagnosis. It is realistic for Ukraine because the institutions of the Ministry of Health of Ukraine have 1,035 endoscopic departments equipped with 1,710 fibrogastroscopes.
Minimally invasive treatment for early GC
The results of GC treatment depend on how advanced the tumor is. Early diagnosis ensures complete curability through surgery; 5-year survival rates after endoscopic mucosectomy are 99%, according to Japanese studies (5-7).
One of the major advances in GC surgery is laparoscopic gastrectomy. A large number of surgeries involving gastrectomy with standard lymph node dissection have been carried out in South Korea and Japan (8,9). The results of randomized research allow us to conclude that gastrectomy has a number of advantages over conventional surgery; these are less blood loss and fewer complications, quicker patient recovery, fewer post-operative bed days, better visual effects, and almost the same 5-year survival rates at 57.3% vs. 58.9% (10-12).
Neo-adjuvant therapy
An increasing number of researchers have stressed the need for combined pre- and post-operative chemo- and radiotherapy for advanced GC. Recent randomized studies in this regard have proved the significant role of pre-operative and adjuvant chemotherapy in the treatment results for patients with operable GC and cancer of the lower thoracic esophagus (13). Meta-analysis of nine randomized studies between 1970 and 2006 (involving 1,700 patients) showed that pre-operative radiotherapy, as well as post-operative chemo- and radiotherapy, considerably increase the 5-year survival rates of such patients (14). As most patients in Ukraine (64%) are diagnosed with GC at stage III–IV, the need to develop and use methods of combined therapy for these patients is particularly urgent.
Surgery
Ukraine has accumulated considerable experience in the field of surgical treatment for GC; it possesses technically accessible, reliable, and efficient methods of performing subtotal and total gastrectomy.
It should also be noted that efficient surgery is particularly important for good remote results of treatment. This involves D2 lymph node dissection and R0 surgery (15). These procedures are possible if surgeons have an approach that allows them to carry out visually controlled surgery of the radical volume (14,16).
The Donetsk Regional Anti-tumor Center uses the following surgical approaches for GC treatment: midline laparotomy, the Lewis approach (midline laparotomy and right thoracotomy), the Garlock approach (left thoracolaparotomy) and an abdomino-mediastinal approach. These approaches enable efficient lymphadenectomy and segmental tumor excision (R0 resection) at different tumor sites.
In order to determine a surgical approach for GC that occupies the esophagus, we rely on the Siewert classification, which is used globally (17). Traditionally, the first type requires a Lewis approach for D2+2F lymphadenectomy. The second and third types need Garlock surgery with D2+2S lymphadenectomy through a left thoracic-abdominal approach. We have found that abdomino-mediastinal approach surgery is possible if the stomach tumor reaches 2 cm into the esophagus. It also requires intra-surgery investigation of the proximal edge of the resection and lymphadenectomy D2+2S; if the edge of the resection contains tumor tissue then a thoracic-abdominal approach is applied.
Over 40 years, our clinic has seen 7,041 radical and palliative GC operations, including 4,381 gastrectomies and 2,910 stomach resections of various types, which has provided the clinic with considerable experience. The post-operative death rate after gastrectomy is 3.3%. The frequency of esophagus anastomosis inefficiency is 0.69% and 0.21% after radical gastrectomy.
During the 3-year period under analysis [2011–2013], the Donetsk Regional Anti-tumor Center performed 1,146 GC surgeries, including 775 radical and palliative operations, and 371 surgical bypasses and explorative laparotomies; this increased the resectability rate to 67.6%. Table 2 presents the classification of patients according to the type of surgery.
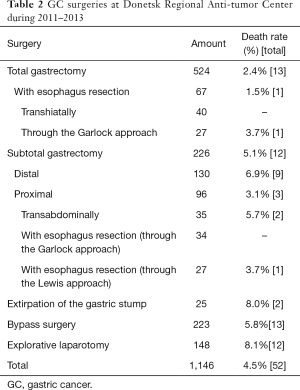
Full table
Most of the patients who underwent surgery were men with a tumor located in the lower one-third of the stomach and a histological structure indicative of adenocarcinoma with varying degrees of differentiation (Table 3).
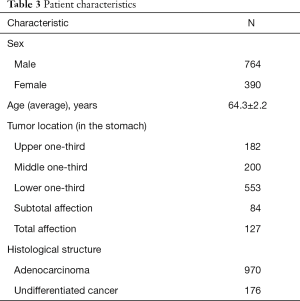
Full table
The post-operative death rate was 4.5% (52 patients).
The functional results of GC surgery showed that the reflux-esophagitis rate was 8%, and the rate of cicatricial stenosis of anastomosis was 6%.
More than 57% of GC patients in Ukraine are diagnosed with advanced and metastatic GC at stage III–IV, and there is often the presence or possibility of complications, such as stenosis, bleeding, or gastric wall perforation. In such cases, only palliative gastrectomy or stomach resection can save the patient’s life and forestall lethal complications (18).
Long-term experience (beginning in 1978) in surgery of the kind performed at Donetsk Regional Anti-tumor Center proves that palliative total and subtotal gastrectomies reinforce the effects of endolymphatic, intra-arterial, and any other chemotherapy. This extends patients’ lives and, to some extent, keeps tumor development in check.
In Ukraine, 56.7% of patients are diagnosed with advanced GC at stage III–IV (T2-4NO-3MO); 20% of these patients develop carcinomatosis of the peritoneum (in Ukraine, this is 1,300 patients annually).
According to various studies, 10–64% of patients have free tumor cells in the peritoneal cavity, which may be the reason for relapse and metastases. The rate of the tumor cell exposure with T3 correlates with the size of the stomach serosa that is affected; this is 10 cm2 in 17.3% of patients and 20 cm2 in 68.5% of patients (19).
Free tumor cells in the peritoneal cavity signify dissemination of the tumor. As a result, surgery is palliative and the patients have a poor prognosis. For this reason, many studies recommend preventive hyperthermochemotherapy (HTCT) for this group of patients. Meta-analysis of the results of randomized studies shows that HTCT doubles the 5-year survival of these patients. For those with resectable stage III GC, their chances of surviving more than 5 years are 4.9 times higher with HTCT than after surgery only. Indications for HTCT are an infiltrative tumor that has spread onto the serosa, disease of T3–4 stage (III or IV growth type according to the Borrmann classification), low-differentiated tumors with spread onto the serosa (T3–4), metastatic affection of the retroperitoneal (celiac) lymph nodes, and the detection of free tumor cells during intraoperative lavage (Sugarbaker P.H., 2003) (19). The given method is suitable for a large number of patients in Ukraine, and can considerably improve the results of treatment. The establishment of centers that could use the described treatments of advanced GC is a topical and realistic task that does not involve significant expense.
Many patients survive for a long time after combined treatment, which makes the problem of surgical prevention of functional complications very topical. The challenge also includes regaining the ability to work and lead a full life for patients who underwent stomach resection and gastrectomy. It gives rise to a new higher level at which the problem of surgical rehabilitation of such patients should be solved (20).
Adjuvant therapy
Combined therapy appears to be very effective for advanced GC. Meta-analysis showed that post-operative chemotherapy increases the 5-year survival of patients who underwent radical surgery (21). It is necessary to implement methods of combined therapy developed by Ukrainian oncologists for advanced GC.
Palliative therapy
The main method of treatment for inoperable and metastatic GC is polychemotherapy (PCT). Randomized studies reveal that PCT is the only way to improve the GC patients’ quality of life, as well as to raise life expectancy from 4–5 to 10–12 months compared with symptomatic treatment (22).
Recent randomized studies have investigated the efficacy of the modern PCT regimens. As compared with the patients treated with the “standard” regimen [cisplatin + 5-fluorouracil (FU)], patients treated with regimens including capecitabine have achieved a median survival of more than 10 months, as well as less toxicity and a better quality of life (23).
Treating patients with HER2-positive GC with trastuzumab (Herceptin) was successful. This type of GC affects 20–30% of all patients, and has a survival rate of 16.0 months (24).
It should be added that the relatively insignificant effect achieved through modern PCT schemes for GC has led to much more expensive treatment (double or triple the costs), which eventually may hamper the extensive implementation of the new regimens in clinical practice.
In this regard, the results of the use of endolymphatic and intra-arterial PCT for unresectable GC should be studied. Studies at Donetsk Regional Anti-tumor Center show that these methods help to achieve a median survival of 8–10 months. They also reduce toxicity (30–50%) and the cost of treatment, which is particularly important. Therefore, we can conclude that inoperable GC needs PCT methods, which combine effectiveness, low toxicity, and affordability on the basis of tailored treatment.
We compared the effectiveness of different methods of PCT in the treatment of 125 patients with unresectable advanced GC. Thirty patients received prolonged intravenous PCT (IVPCT) under the PF regimen (cisplatin—100 mg/m2 intravenously, 5-FU—1,000 mg/m2 per day intravenously, 120 hours—continuous infusion). Forty-five patients received intralymphatic PCT (ILPCT) in the same way as PF (cisplatin—100 mg/m2 intravenously, 5-FU—1,000 mg/m2 per day intralymphatic, 5 days), and 50 patients received intra-arterial chemotherapy (IAPCT) after exploratory laparotomy. Patients from the IAPCT group received 1–6 courses of CT in continuous prolonged intra-arterial infusion mode using SB-07 or UN2/50; this involved 5-FU at 10–15 mg/kg/day, every day as a continuous infusion over 10–12 hours with 3–5 mL per hour. The duration of each course was 5 days with a 21-day interval between courses. All three groups of patients were comparable in key parameters (Table 4).
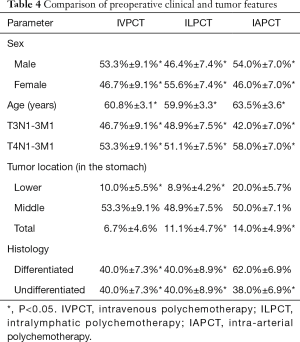
Full table
Among the 45 patients who received ILPCT, the efficacy of treatment according to RECIST was proved for 32 (71.1%±6.8%) patients with measurable metastasis to the liver, retroperitoneal lymph nodes, or lungs. Five patients died within the first 1–2 months of observation following clinical deterioration of their general condition. A complete response was not observed in any of the 32 patients. A partial response was observed in 9 (28.1%±7.9%) of the 32 patients, all of whom received 5 or more courses of ILPCT. Stabilization of the tumor was recorded in 11 (34.4%±8.4%) patients who received 3–4 courses of PCT; the remaining 12 patients (37.5%±8.6%), who received 1–2 courses of PCT, showed disease progression. Thirty patients received standard prolonged IVPCT; the efficacy of treatment according to RECIST was observed in all 30 patients. A complete response was not observed in any of these patients. A partial response was recorded in 11 (36.7%±8.8%) patients after 4–6 courses of IVPCT. Stabilization of the tumor occurred in 9 (30.0%±8.4%) patients who received 3–5 courses of PCT. Ten patients (33.3%±8.6%) experienced disease progression shortly after the start of treatment and therefore, they only received 1–2 courses of PCT.
We evaluated 50 patients who received IAPCT for unresectable GC; the results showed that catheterization of the right gastroepiploic artery during laparotomy for unresectable GC does not increase the morbidity of postoperative complications and postoperative mortality.
The immediate effect of the treatment, as determined using RECIST, was evaluated in 42 (84%) out of 50 patients. A complete response was not observed in any of the patients; however, a partial response was recorded in 16 (38.1%±7.5%) patients after 3–6 courses of IAPCT. Stabilization of the tumor was observed in 15 (35.7%±5.4%) patients; they received 3–4 courses of PCT. Eleven patients (26.2%±6.8%) demonstrated disease progression shortly after the start of treatment and thus, they only received 1–2 courses of PCT.
The median survival times with various chemotherapy combinations were different; the median survival was 8.1 months after treatment with ILPCT, 9.2 months after treatment with IVPCT, and 10.8 months after treatment with IAPCT. The difference being statistically unreliable, the differences between long-term results of treatment in studied groups of patients depending on the localization of distant metastases were analyzed. In this analysis, it was found that the survival rate in patients receiving ILPCT varies depending on the location of metastatic lesions. Primary carcinomatosis of the parietal peritoneum and spread to the retroperitoneal lymph nodes was associated with significantly higher median survival than the development of distant metastases in the liver and pancreas. This confirms published data that shows that, during intralymphatic administration of chemotherapy, the maximum drug concentration is reached in the lymph collectors of the abdomen and retroperitoneum.
For the analysis of the results, we divided patients with unresectable GC, according to the localization of distant metastases into two groups; these consisted of patients with a primary lesion in the parietal peritoneum and retroperitoneal lymph nodes, and those with a primary lesion in the liver and pancreas. When comparing survival in patients with unresectable GC treated using ILPCT (45 patients), IAPCT (50 patients), and prolonged IVPCT (30 patients), it was found that ILPCT resulted in a median survival of 11.6 months for patients with a primary lesion of the parietal peritoneum and retroperitoneal lymph nodes. The same method resulted in a median survival of 6.5 months for patients with a metastatic lesion in the liver and pancreas. A higher survival in patients with metastases in the liver and pancreas (12.4 vs. 4.5 months) was achieved using IAPCT. Patients treated with IVPCT had the same (8.9 vs. 8.8 months) survival rates in both groups.
Conclusions
One of the unsolved challenges of nationwide importance is the early diagnosis of GC, which predetermines the treatment results. The 5-year survival rate for GC patients in Ukraine is only 13%, while early diagnosed GC is almost totally curable though surgery.
Despite sufficient technical provision, 70% of Ukrainian patients are diagnosed with GC at stage III–IV. Significant changes are only possible if patients are diagnosed with GC at an early stage; this requires an efficient system of early diagnosis that will also necessitate the patients’ involvement.
Another important task is the steady development of national diagnosis and treatment standards, which, based on national breakthroughs, would meet modern international requirements. Commitment of both the state and oncological clinics is required to guarantee modern oncological treatment for the entire Ukrainian population, as well as to encourage steady improvements in treatment quality.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Goyang: National Cancer information Center. Available online: http://www.cancer.go.kr/mbs/cancer/subview.jsp?id=cancer_040101000000
- Daejeon: Korean Statistical Information Service. Available online: http://kosis.kr/index/index.jsp
- Mocellin S, Marchet A, Nitti D. EUS for the staging of gastric cancer: a meta-analysis. Gastrointest Endosc 2011;73:1122-34. [Crossref] [PubMed]
- Murakami R, Tsukuma H, Ubukata T, et al. Estimation of validity of mass screening program for gastric cancer in Osaka, Japan. Cancer 1990;65:1255-60. [Crossref] [PubMed]
- Choi KS, Jung HY, Choi KD, et al. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc 2011;73:942-8. [Crossref] [PubMed]
- Chiu CC. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria (Br J Surg 2010; 97: 868-871). Br J Surg 2010;97:1455; author reply 1455-6. [Crossref] [PubMed]
- Bennett C, Wang Y, Pan T. Endoscopic mucosal resection for early gastric cancer. Cochrane Database Syst Rev 2009.CD004276. [PubMed]
- Kitano S, Shiraishi N, Uyama I, et al. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg 2007;245:68-72. [Crossref] [PubMed]
- Hwang SH, Park DJ, Jee YS, et al. Actual 3-year survival after laparoscopy-assisted gastrectomy for gastric cancer. Arch Surg 2009;144:559-64. [Crossref] [PubMed]
- Fujiwara M, Kodera Y, Misawa K, et al. Longterm outcomes of early-stage gastric carcinoma patients treated with laparoscopy-assisted surgery. J Am Coll Surg 2008;206:138-43. [Crossref] [PubMed]
- Jiang X, Hiki N, Nunobe S, et al. Long-term outcome and survival with laparoscopy-assisted pylorus-preserving gastrectomy for early gastric cancer. Surg Endosc 2011;25:1182-6. [Crossref] [PubMed]
- Song J, Lee HJ, Cho GS, et al. Recurrence following laparoscopy-assisted gastrectomy for gastric cancer: a multicenter retrospective analysis of 1,417 patients. Ann Surg Oncol 2010;17:1777-86. [Crossref] [PubMed]
- An JY, Kim HI, Cheong JH, et al. Pathologic and oncologic outcomes in locally advanced gastric cancer with neoadjuvant chemotherapy or chemoradiotherapy. Yonsei Med J 2013;54:888-94. [Crossref] [PubMed]
- Lee JH, Kim KM, Cheong JH, et al. Current management and future strategies of gastric cancer. Yonsei Med J 2012;53:248-57. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23. [Crossref] [PubMed]
- Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 2008;359:453-62. [Crossref] [PubMed]
- Siewert JR, Stein HJ, Feith M. Adenocarcinoma of the esophago-gastric junction. Scand J Surg 2006;95:260-9. [Crossref] [PubMed]
- Sarela AI, Yelluri S. Leeds Upper Gastrointestinal Cancer Multidisciplinary Team. Gastric adenocarcinoma with distant metastasis: is gastrectomy necessary? Arch Surg 2007;142:143-9. [Crossref] [PubMed]
- Glehen O, Mithieux F, Osinsky D, et al. Surgery combined with peritonectomy procedures and intraperitoneal chemohyperthermia in abdominal cancers with peritoneal carcinomatosis: a phase II study. J Clin Oncol 2003;21:799-806. [Crossref] [PubMed]
- Cheong JH, Hyung WJ, Chen J, et al. Survival benefit of metastasectomy for Krukenberg tumors from gastric cancer. Gynecol Oncol 2004;94:477-82. [Crossref] [PubMed]
- GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group, Paoletti X, Oba K, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA 2010;303:1729-37. [Crossref] [PubMed]
- Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006;24:2903-9. [Crossref] [PubMed]
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
Cite this article as: Sydiuk A. Current practice for gastric cancer treatment in Ukraine. Transl Gastroenterol Hepatol 2017;2:47.


