Laparoscopic endoscopic cooperative surgery (LECS) for the upper gastrointestinal tract
Introduction
We developed the laparoscopic endoscopic cooperative surgery (LECS) technique, which combines endoscopic submucosal dissection (ESD) and laparoscopic gastric resection to resect gastric submucosal tumors (SMTs). Since we first reported LECS in 2008 (1), many researchers have attempted this procedure (2-8) and reported that LECS is a feasible procedure for gastric SMTs that can be used regardless of tumor location. Recently, the Japanese National Health Insurance system approved LECS for insurance coverage, and it is now widely applied for gastric submucosal tumor resection. Before we developed LECS, laparoscopic wedge resection was generally used for the surgical treatment of gastric SMT. However, the ideal resection line is not easy to determine with only a laparoscopic view, especially for intragastric SMTs, as normal-appearing gastric wall covers these tumors. Therefore, postoperative transformation of the stomach may be caused by excessive gastric resection, with consequent gastric stasis and the possibility of local recurrence reported due to an inappropriate resection line (2,9).
An advantage of LECS is the completeness of the resection with minimal margin. Using the LECS procedure, the gastric wall, feeder vessels, and nerves can be preserved, which in turn preserves gastric function and improves the patient’s postoperative quality of life.
We first applied LECS to gastric SMTs without ulcerative lesions due to concern about the possibility of tumor cell seeding into the peritoneal cavity. In this procedure, termed “classical LECS”, we limited the maximum tumor diameter to 50 mm in accordance with the indications for laparoscopic resection of gastrointestinal stromal tumors (GISTs) that have been proposed by the National Comprehensive Cancer Network.
Preventing tumor seeding and the contamination of gastric juice in the peritoneal cavity are important considerations when performing LECS for gastric epithelial neoplasms. To expand the indication of LECS for gastric epithelial neoplasms, some modified LECS procedures such as inverted LECS (10), non-exposed endoscopic wall-inversion surgery (NEWS) (11-15), combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure technique (CLEAN-NET) (16), and closed laparoscopic and endoscopic cooperative surgery (closed-LECS) (17) have been developed and applied to patients with gastric epithelial neoplasms.
Here, we discuss the practical application of classical LECS for gastric SMTs, review the existing modified LECS procedures for gastric epithelial neoplasms, and discuss future perspectives of the technique.
Classical LECS for gastric SMT
Procedure for classical LECS (1,2)
The operating surgeon is positioned at the patient’s right side, the first assistant at the patient’s left side, the laparoscopist at the patient’s foot side, and the endoscopist stood near the patient’s head side. A camera port is inserted into the umbilicus. Four additional ports (one 12-mm port and three 5-mm ports) are placed at the right upper, right lower, left upper, and left lower quadrants with visual assistance of the laparoscopic image under 10-mmHg pneumoperitoneum.
The location of the tumor is determined by intraluminal endoscopy and laparoscopic view. Blood vessels around the tumor are prepared using a vessel-sealing system or ultrasonically activated device. The area of the blood vessels and nerve manipulation should be minimized to prevent postoperative gastric stasis and ischemia. We reported a case of suture line leakage by ischemia caused by excessive vascular preparation (2).
The circumference of the tumor is marked on the tumor edge with a 0.5-cm margin. Next, 10% glycerin is injected into the submucosal layer, a small initial cut is made using a standard needle knife, and the point of an IT-2 knife (KD-611L; Olympus, Tokyo, Japan) is inserted into the submucosal layer. The premarked area is cut circumferentially using an IT-2 knife. Then a standard needle knife is pushed toward the serosa. The point of the standard needle knife is seen on the laparoscopic image under the seromuscular layer and is used to perforate the seromuscular layer. The seromuscular dissection is started from the perforation point using an IT-2 knife or ultrasonically activated device. The seromuscular layer is cut along the submucosal incision line. The proper resection line can be easily confirmed, because we can observe the resection line both laparoscopically and endoscopically. The seromuscular incision is made by an endoscope device with laparoscopic assistance as much as possible, and the remaining part of the dissection is performed laparoscopically. The defect of the gastric wall is closed temporarily using hand-sewn sutures after the tumor is resected. Then a laparoscopic stapling device is used to close the incision line. The tumor is transferred into a collection bag and removed. Finally, we confirm the absence of air leaks at the stapling line by endoscopic insufflation, and the absence of bleeding is confirmed both endoscopically and laparoscopically.
Advantages and limitations of classical LECS
Many researchers have reported that classical LECS is a feasible and safe surgical procedure for the treatment of gastric SMTs (Table 1). The benefit of classical LECS is the completeness of the resection with a minimal margin. Classical LECS is technically easier than the modified LECS procedures. Thus, classical LECS can be applied to any tumor location including the EGJ. We can make the best use of the advantages of LECS for gastric SMTs located at the EGJ by avoiding conventional total gastrectomy or proximal gastrectomy. Hoteya et al. reported the feasibility of classical LECS for gastric SMTs located at the EGJ (4).

Full table
A limitation of classical LECS is the possibility of tumor and gastric juice contamination into the abdominal cavity due to opening of the gastric wall during the procedure. Accordingly, classical LECS can be applied to gastric SMTs without a mucosal defect.
Modified LECS procedures for gastric epithelial neoplasms
Inverted LECS
We developed the inverted LECS technique to avoid contamination of gastric juice or direct contact between the surrounding tissue and the tumor (10). After determining the resection line by endoscopic mucosal incision, the stomach wall around the tumor is sutured and pulled up like a bowl. Each of the stitches is pulled out of the abdominal cavity using the Endo CloseTM site closure device (Covidien, Tokyo, Japan) and fixed at skin level. The full-thickness incision is made endoscopically and laparoscopically. During full-thickness incision, the tumor is inverted into the gastric cavity to prevent gastric juice contamination and direct contact between the abdominal cavity and tumor. The tumor is then resected, dropped into the gastric cavity, and collected via the per-oral route.
This technique theoretically minimizes the risk of gastric content contamination into the abdominal cavity and prevents direct contact between the tumor and any intra-abdominal tissue. Furthermore, inverted LECS is less complicated than other modified LECS procedures. However, this method also requires opening of the gastric wall during the procedure. Thus, a slight risk of tumor dissemination or abdominal abscess cannot be ruled out.
NEWS
NEWS has been reported as a novel full-thickness resection technique without gastric perforation, aimed mainly at treating early gastric cancer (11-15). In this procedure, mucosal markings are first placed around the tumor, followed by serosal markings via laparoscope under endoscopic navigation. Then, sodium hyaluronate solution containing indigo carmine dye is injected into the submucosal layer circumferentially by endoscope. A circumferential seromuscular incision is performed laparoscopically around the serosal markings. The seromuscular layers are sutured, and the lesion is inverted into the inside of the stomach. During suturing, a laparoscopic surgical sponge is inserted to create a space between the suture layer and the serosal layer of the inverted lesion. This spacer provides a counter-traction to the mucosa and protects the suture line during the subsequent endoscopic resection. Finally, circumferential mucosal and submucosal tissue incisions are made endoscopically around the inverted tumor. The resected tumor and the spacer are retrieved perorally, and the mucosal defect is sutured with several endoscopic clips.
As an advantage of this technique, both the serosal and mucosal layers can be resected precisely under direct visualization by laparoscopy or endoscopy. Its major limitation is the size of the tumor. NEWS is indicated for gastric SMTs 30 mm or less in diameter because the resected specimen is retrieved perorally.
CLEAN-NET
Inoue et al. (16) developed a method of non-exposed full-thickness resection after seromuscular incision, preserving the mucosa, which plays a role as a barrier. They refer to this technique as CLEAN-NET. In this technique, after endoscopic marking, four full-layer stay sutures fix the mucosal layer to the seromuscular layer. The seromuscular layer is dissected laparoscopically along the outside of the stay sutures. The specimen is pulled up by the stay sutures, and the mucosa surrounding the specimen is also lifted. The continuity of the mucosal layer prevents the gastric contents from flowing out into the peritoneal cavity. The full-layer specimen is resected with an adequate surgical margin using a laparoscopic stapling device.
CLEAN-NET is a unique procedure and an attractive non-exposure technique for full-thickness resection of the gastric wall. However, if the tumor is located at the cardia, CLEAN-NET might be difficult to perform. Furthermore, in this procedure, the incision line is ultimately decided on from the serosal side. Therefore, compared to other LECS modified procedures, determining the appropriate resection line might be difficult.
Closed-LECS
Kikuchi et al. (17) developed a new non-exposed full-thickness resection called “closed LECS”. In this procedure, mucosal markings are first made at the periphery of the tumor, and the marked circumferential area is cut endoscopically. Then serosal markings are made corresponding to the submucosal dissection line using the guide of endoscopic light. Next, a spongy spacer is placed at the center of the suture line on the serosal surface, and a seromuscular suture is made, with inversion of the marked lesion and spacer into the inside of the stomach. Finally, circumferential seromuscular dissection is performed endoscopically. The resected specimen and sponge spacer are retrieved perorally.
The closed LECS procedure is advantageous with regard to determining the appropriate resection line because the resection line is determined by endoscopy, as in the classical LECS and inverted LECS procedures. The limitation of this method is tumor size, as closed LECS is indicated for gastric SMTs 30 mm or less in diameter because the resected specimen is retrieved perorally.
Future perspectives for LECS procedures
As we described, LECS can be applied to patients with gastric SMTs safely, and modified LECS procedures (inverted LECS, NEWS, CLEAN-NET and closed-LECS) have been developed primarily aimed at gastric epithelial neoplasms.
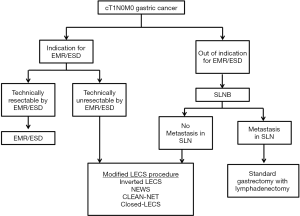
At this time, modified LECS procedures can be applied for cases of early gastric cancer [within the indication for endoscopic mucosal resection (EMR)/ESD] that would be technically difficult to treat with EMR/ESD. If the tumor is beyond the indication for EMR/ESD, the case involves the possibility of lymph node metastasis, and gastrectomy with lymphadenectomy must be performed.
Goto et al. reported NEWS for early gastric cancer in combination with sentinel node (SN) navigation surgery (SNNS) (12,18). The SN is the first lymph node that receives lymphatic drainage from the primary tumor. Thus, we can predict the pathological status of all other lymph nodes from the SN status. If the SNs are detected and confirmed to be cancer metastasis-negative, then unneeded lymph node dissection may be avoided. SNNS for gastric cancer has been validated in a prospective multicenter trial (19). In addition, a prospective multicenter trial is ongoing to compare personalized gastrectomy with conventional distal/total gastrectomy, based on intraoperative SN biopsy data. When modified LECS procedures and SNNS are performed together, we can provide a minimally invasive surgical technique for cStage IA gastric cancer (Figure 1).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hiki N, Yamamoto Y, Fukunaga T, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc 2008;22:1729-35. [Crossref] [PubMed]
- Matsuda T, Hiki N, Nunobe S, et al. Feasibility of laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors (with video). Gastrointest Endosc 2016;84:47-52. [Crossref] [PubMed]
- Tsujimoto H, Yaguchi Y, Kumano I, et al. Successful gastric submucosal tumor resection using laparoscopic and endoscopic cooperative surgery. World J Surg 2012;36:327-30. [Crossref] [PubMed]
- Hoteya S, Haruta S, Shinohara H, et al. Feasibility and safety of laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors, including esophagogastric junction tumors. Dig Endosc 2014;26:538-44. [Crossref] [PubMed]
- Obuchi T, Sasaki A, Baba S, et al. Single-port laparoscopic and endoscopic cooperative surgery for a gastric gastrointestinal stromal tumor: report of a case. Surg Today 2015;45:641-6. [Crossref] [PubMed]
- Waseda Y, Doyama H, Inaki N, et al. Does laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors preserve residual gastric motility? Results of a retrospective single-center study. PLoS One 2014;9:e101337. [Crossref] [PubMed]
- Qiu WQ, Zhuang J, Wang M, et al. Minimally invasive treatment of laparoscopic and endoscopic cooperative surgery for patients with gastric gastrointestinal stromal tumors. J Dig Dis 2013;14:469-73. [Crossref] [PubMed]
- Kawahira H, Hayashi H, Natsume T, et al. Surgical advantages of gastric SMTs by laparoscopy and endoscopy cooperative surgery. Hepatogastroenterology 2012;59:415-7. [Crossref] [PubMed]
- Hu J, Or BH, Hu K, et al. Comparison of the post-operative outcomes and survival of laparoscopic versus open resections for gastric gastrointestinal stromal tumors: A multi-center prospective cohort study. Int J Surg 2016;33 Pt A:65-71.
- Nunobe S, Hiki N, Gotoda T, et al. Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for a lateral-spreading mucosal gastric cancer. Gastric Cancer 2012;15:338-42. [Crossref] [PubMed]
- Goto O, Mitsui T, Fujishiro M, et al. New method of endoscopic full-thickness resection: a pilot study of non-exposed endoscopic wall-inversion surgery in an ex vivo porcine model. Gastric Cancer 2011;14:183-7. [Crossref] [PubMed]
- Goto O, Takeuchi H, Kawakubo H, et al. First case of non-exposed endoscopic wall-inversion surgery with sentinel node basin dissection for early gastric cancer. Gastric Cancer 2015;18:434-9. [Crossref] [PubMed]
- Mitsui T, Goto O, Shimizu N, et al. Novel technique for full-thickness resection of gastric malignancy: feasibility of nonexposed endoscopic wall-inversion surgery (news) in porcine models. Surg Laparosc Endosc Percutan Tech 2013;23:e217-21. [Crossref] [PubMed]
- Mitsui T, Niimi K, Yamashita H, et al. Non-exposed endoscopic wall-inversion surgery as a novel partial gastrectomy technique. Gastric Cancer 2014;17:594-9. [Crossref] [PubMed]
- Goto O, Takeuchi H, Kawakubo H, et al. Feasibility of non-exposed endoscopic wall-inversion surgery with sentinel node basin dissection as a new surgical method for early gastric cancer: a porcine survival study. Gastric Cancer 2015;18:440-5. [Crossref] [PubMed]
- Inoue H, Ikeda H, Hosoya T, et al. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond: full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET). Surg Oncol Clin N Am 2012;21:129-40. [Crossref] [PubMed]
- Kikuchi S, Nishizaki M, Kuroda S, et al. Nonexposure laparoscopic and endoscopic cooperative surgery (closed laparoscopic and endoscopic cooperative surgery) for gastric submucosal tumor. Gastric Cancer 2017;20:553-7. [Crossref] [PubMed]
- Goto O, Takeuchi H, Kitagawa Y, et al. Hybrid surgery for early gastric cancer. Transl Gastroenterol Hepatol 2016;1:26. [Crossref] [PubMed]
- Kitagawa Y, Takeuchi H, Takagi Y, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol 2013;31:3704-10. [Crossref] [PubMed]
Cite this article as: Matsuda T, Nunobe S, Ohashi M, Hiki N. Laparoscopic endoscopic cooperative surgery (LECS) for the upper gastrointestinal tract. Transl Gastroenterol Hepatol 2017;2:40.


