Recent trends of gastric cancer treatment in Turkey
Approximately one million new cases of stomach cancer were estimated to have occurred in 2012 (952,000 cases, 6.8% of the total) according to the World Health Organization (WHO)-International Agency for Research on Cancer, making it the fifth most common malignancy in the world, after lung, breast, colorectum, and prostate cancer. More than 70% of cases occur in developing countries, and half the global cases occur in Eastern Asia. Moreover, regarding mortality, stomach cancer is the third leading cause of death due to cancer worldwide. Eastern Asia is associated with the highest estimated mortality rates, while the lowest are in Northern America (1).
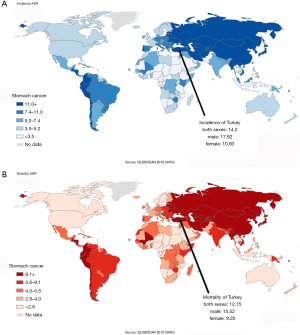
In Turkey, gastric cancer is the fifth most common type of cancer and is the fourth most common death due to cancer. Incidence and mortality rates are one of the highest in Europe, with a 14.20 per 100,000 incidence rate and 12.15 per 100,000 mortality rate (Figure 1) (1). Turkey, with a population of nearly 80 million, is a country associated with one of the highest numbers of gastric cancer cases among all European countries. Additionally, in Turkey, there is some variability in the incidence rate between regions, with the East exhibiting a greater number of gastric cancer cases compared to the West (2,3).
Despite the high incidence rate, management of gastric cancer is still an issue of debate in Turkey and national guidelines have yet to be established. Moreover, no standardized training program for the management of gastric cancer has been established, to date. Treatment approaches are decided based on the individual origin of each specialist’s training. Thus, physicians who were trained in the west, follow the European or National Comprehensive Cancer Network (NCCN) guidelines, while physicians with experience in Japan or South Korea follow the eastern guidelines. Due to the lack of a consensus regarding the management of gastric cancer in Turkey, it is quite difficult to present the current practice for the entire country. Therefore, this review will focus on the recent trends of gastric cancer treatment (except for esophagogastric junction tumors) in Turkey by exploring the institutional approach.
Pre-treatment evaluation
Diagnosis of gastric cancer is mainly confirmed by an upper gastrointestinal endoscopy and biopsy and is reported according to the WHO criteria (4). When an endoscopic biopsy cannot confirm malignancy despite a high suspicion of gastric cancer (e.g., Borrmann type-IV cancer), histological confirmation is achieved with a surgical biopsy via an open or laparoscopic approach. Since the treatment plan following confirmation of the diagnosis is based on the clinical stage of the tumor, the clinical condition of the patient and the patient’s symptoms, the greatest effort is focused on proper evaluation, particularly to determine the appropriate staging. To this end, contrast-enhanced thoracoabdominal multidetector-row computed tomography (CT) is used for all patients. Some additional staging modalities are used for accurate pre-treatment staging situations, such as abdomen magnetic resonance imaging for evaluation of liver metastasis, positron emission tomography-CT for evaluation of systemic lymph node or distant metastasis, and diagnostic laparoscopy for the suspicion of peritoneal metastasis (5). Since the incidence of early gastric cancer is low and endoscopic treatment is not common in Turkey, endoscopic sonography is not preferred in most centers.
Treatment strategy
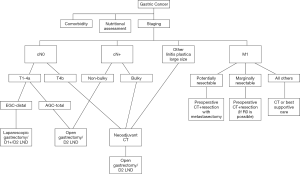
While the treatment plan following the diagnosis of gastric cancer is determined by a multidisciplinary meeting at high-volume centers, the plan in the majority of cases in Turkey is decided based on the decision of an individual physician (6). The primary goal of the treatment strategy is to obtain the best oncological outcomes and quality of life within the acceptable treatment-related morbidity. Thus, R0 resection is intended by using standardized surgery with an individualized approach, while avoiding surgery if a curative resection is not possible (except in patients with bleeding or an obstruction). The algorithm in our institute is summarized in Figure 2.
Management of locoregional disease
Endoscopic treatment: Endoscopic resection techniques used in Japan and South Korea for early gastric cancer may not become common in western countries due to the scarcity of early gastric cancer. In Turkey, the early gastric cancer ratio is lower than 10% among all gastric cancer cases and exhibits a parallel trend to other European countries (7,8). Although there are centers in Turkey that successfully perform endoscopic resections for cancer, it is challenging to increase the case volume due to the lack of a screening program for gastric cancer.
Surgical treatment: Surgical resection is a potentially curative treatment for gastric cancer, especially when diagnosed in earlier stages. However, due to problems (e.g., older population, delayed diagnosis, advanced disease, nutritional problems before treatment, cancer cachexia, and possibility of cancer recurrence despite the curative treatment), the preferred surgical strategy is not judged only as a technical event but is valued as a sophisticated process.
In our practice, a detailed preoperative nutritional assessment is performed to determine the potential malnutrition in patients with gastric cancer (9,10). In addition to simple anthropometric measurements (e.g., weight, body mass index, weight loss ratio, and Nutritional Risk Screening (NRS)-2002 form), we evaluate the sarcopenia with muscle mass volume using an abdominal CT, muscle strength using a handgrip strength test, and physical performance via a speed test. For patients exhibiting a 10–15% weight loss in the past three to six months, Body mass index <18 kg/m2, NRS-2002 score >4, and albumin level < 3 g/dL are classified as severe malnutrition in accordance with the European Society for Clinical Nutrition and Metabolism guidelines (11). Thus, we initiate preoperative nutritional support for this group of patients, even if surgery must be delayed. For patients exhibiting no malnutrition or mild to moderate malnutrition, we do not use preoperative nutritional treatment and do not postpone the surgery. In addition to the standard nutritional treatment, we prefer to use enteral immunonutrition mixtures containing glutamine and arginine for five to seven days (11,12).
The extent of gastrectomy is determined according to the tumor location, and a total or distal gastrectomy is performed in the majority of cases. Proximal gastrectomy and pylorus-preserving gastrectomy are techniques that are rarely performed. A 3–5 cm resection margin is accepted as sufficient based on the histopathological features of the tumor; however, when this rule cannot be respected, we prefer to examine the proximal resection margin using a frozen section (13).
The primary debate for surgical treatment pertains to the extent of the lymphadenectomy accompanying a radical gastrectomy. Similar to other countries throughout the world, except for East Asian and some European countries, Turkey does not have a national consensus regarding the extent of the lymphadenectomy. While many centers still consider a D1 dissection to be sufficient, specialized centers and surgeons prefer the D2 Dissection as a standard surgical strategy. Although the benefits of a D2 dissection, especially in advanced stages is well known, the lack of experience for D2 lymphadenectomy and high morbidity/mortality rates of previous western studies prevent surgeons from performing extensive surgery (14-16). Similar debates are ongoing for bursectomy; however, surgeons performing a routine D2 dissection prefer the routine bursectomy for all patients based on their traditional habits. At our institute, we only perform complete bursectomy for serosa-positive tumors located on the posterior wall of the stomach (17,18). We believe that outcomes of Japanese study (JCOG 1001) which has completed accrual, will determine our future approach. Some images from our patients were presented in Figure 3.
Minimally invasive surgery is common in Turkey similar to the worldwide trend and is used for the treatment of various benign and malignant diseases. Laparoscopic and robotic surgery used for gastrointestinal cancer surgery especially colorectal cancer, is also used for the treatment of gastric cancer in Turkey (19,20). However, in our experience, due to the low incidence of early cancer and that the majority of patients have T4 or cN+ disease, using a minimally invasive approach could not become the standardized approach, and we prefer using them primarily for early cancers particularly requiring distal gastrectomy. We perform laparoscopic gastrectomy with a D2 dissection with totally intracorporeal anastomosis less frequently for advanced disease and for the patients requiring a total gastrectomy. We expect to use more widespread practice of minimally invasive surgery after the outcomes of Japanese and Korean studies (21,22). In addition to the widespread use of laparoscopic surgery, there are currently 34 robotic systems available in Turkey that are predominantly preferred for urological surgery; however, these are not yet common for the practice of general surgery.
Adjuvant/Neoadjuvant treatment: The decision for adjuvant treatment following a gastrectomy is mostly based on the NCCN guidelines in Turkey (5). Therefore, adjuvant chemoradiation is the standard treatment for patients having >T1 or pN+. However, recent advances (e.g., the outcomes of the ARTIST trial and recent versions of the NCCN guidelines indicating the possibility of adjuvant chemotherapy only following a D2 dissection) have led us to shift our management strategy for adjuvant treatment (23,24). Some centers still continue to use adjuvant chemoradiation while others prefer only chemotherapy if a D2 dissection is performed. At our institute, we prefer to use adjuvant chemoradiation for all node positive diseases, even after a D2 dissection, while chemotherapy-only is used for node negative >T1 disease based on the data from of subgroup analysis of the ARTIST trial. The outcomes of the ARTIST-II trial will help us to determine the suitability of our approach (25).
By administering chemotherapy preoperatively, the potential benefits including tumor down-staging, eliminating micrometastases, and determining whether the tumor is sensitive to the chemotherapy are expected. Previous studies have demonstrated that the overall and progression-free survival advantages of perioperative chemotherapy for T2 or higher stage tumors and perioperative treatment became the standard approach in the majority of western countries (26,27). In our practice, we selectively use perioperative treatment even for lesions that are initially resectable to increase the chance of a R0 resection and to ensure the early systemic control of cancer. Current indications for neoadjuvant treatment in our practice include the presence of a T4b tumor, bulky lymph nodes, linitis plastica, and endoscopically large tumors that longitudinally enclose at least two-thirds of the stomach (28,29).
Management of metastatic disease
When the disease is diagnosed at a metastatic stage, treatment options consist of only palliative chemotherapy or best supportive care and surgical treatment is limited to only tumor complications. However, data from the eastern experience and the possibility of more extensive surgeries with low morbidity have led the physicians to be more aggressive in the treatment of metastatic disease. Curative outcomes have been reported with an R0 resection in potentially/marginally resectable cases, although they are initially metastatic diseases, referred to as conversion treatment (30-32). Although potentially resectable cases, such as single liver metastasis or 16a2-b1 paraaortic lymph node metastasis are technically suitable for surgical resection, we prefer preoperative chemotherapy followed by surgical resection. During surgery, we perform a metastasectomy (or paraaortic lymph node dissection) in addition to the radical gastrectomy with D2 lymphadenectomy with curative intent. For patients defined as marginally resectable (e.g., liver metastasis >1 in number or >5 cm in size, extraabdominal lymph node involvement), we commence treatment with intensive chemotherapy, and we evaluate the response. In the case of a partial or complete response, we perform radical surgery if an R0 resection is possible. We sometimes use radiofrequency ablation treatment for synchronous or metachronous liver metastasis when they are not suitable for surgery (33). The main obstacle to patient selection in conversion therapy is the presence of macroscopic peritoneal metastases. Under such circumstances, surgical treatment is used only for palliative intent, and treatment is continued only with chemotherapy or the best supportive care.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. Available online: http://globocan.iarc.fr
- Fatih A, Yasin O, Hakan D, et al. Should every region use the same gastric cancer scanning and treatment approaches? let's reconsider: a northeastern turkey example. BMC Gastroenterol 2016;16:120. [Crossref] [PubMed]
- Basaran H, Koca T, Cerkesli AK, et al. Treatment outcomes and survival study of gastric cancer patients: a retrospective analysis in an endemic region. Asian Pac J Cancer Prev 2015;16:2055-60. [Crossref] [PubMed]
- Waddell T, Verheij M, Allum W, et al. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol 2014;40:584-91. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1286-312. [Crossref] [PubMed]
- Yalcin S, Gumus M, Kilickap S, et al. End-of-study results of Turkish gastric cancer patients from the global REGATE study. J BUON 2014;19:377-87. [PubMed]
- Aslan F, Alper E, Cekıc C, et al. Endoscopic submucosal dissection in gastric lesions: the 100 cases experience from a tertiary reference center in West. Scand J Gastroenterol 2015;50:368-75. [Crossref] [PubMed]
- Hulagu S, Senturk O, Aygun C, et al. Endoscopic submucosal dissection for premalignant lesions and noninvasive early gastrointestinal cancers. World J Gastroenterol 2011;17:1701-9. [Crossref] [PubMed]
- Sakurai K, Ohira M, Tamura T, et al. Predictive Potential of Preoperative Nutritional Status in Long-Term Outcome Projections for Patients with Gastric Cancer. Ann Surg Oncol 2016;23:525-33. [Crossref] [PubMed]
- Fukuda Y, Yamamoto K, Hirao M, et al. Prevalence of Malnutrition Among Gastric Cancer Patients Undergoing Gastrectomy and Optimal Preoperative Nutritional Support for Preventing Surgical Site Infections. Ann Surg Oncol 2015;22 Suppl 3:S778-85. [Crossref] [PubMed]
- Weimann A, Braga M, Harsanyi L, Laviano A, et al. ESPEN Guidelines on Enteral Nutrition: Surgery including organ transplantation. Clin Nutr 2006;25:224-44. [Crossref] [PubMed]
- Braga M, Wischmeyer PE, Drover J, et al. Clinical evidence for pharmaconutrition in major elective surgery. JPEN J Parenter Enteral Nutr 2013;37:66S-72S. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1-19. [Crossref] [PubMed]
- van de Velde CJ. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer (Br J Surg 2014; 101: 23-31). Br J Surg 2014;101:31-2. [Crossref] [PubMed]
- Cuschieri A, Fayers P, Fielding J, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet 1996;347:995-9. [Crossref] [PubMed]
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [Crossref] [PubMed]
- Fujita J, Kurokawa Y, Sugimoto T, et al. Survival benefit of bursectomy in patients with resectable gastric cancer: interim analysis results of a randomized controlled trial. Gastric Cancer 2012;15:42-8. [Crossref] [PubMed]
- Kochi M, Fujii M, Kanamori N, et al. D2 gastrectomy with versus without bursectomy for gastric cancer. Am J Clin Oncol 2014;37:222-6. [Crossref] [PubMed]
- Güner A, Hyung WJ. Minimally invasive surgery for gastric cancer. Ulus Cerrahi Derg 2013;30:1-9. [PubMed]
- Alimoğlu O, Atak İ, Eren T, et al. Robot assisted laparoscopic (RAL) gastrectomy: case series and a review of the literature. Ulus Cerrahi Derg 2013;29:187-91. [PubMed]
- Son T, Kwon IG, Hyung WJ. Minimally invasive surgery for gastric cancer treatment: current status and future perspectives. Gut Liver 2014;8:229-36. [Crossref] [PubMed]
- Inaki N, Etoh T, Ohyama T, et al. A Multi-institutional, Prospective, Phase II Feasibility Study of Laparoscopy-Assisted Distal Gastrectomy with D2 Lymph Node Dissection for Locally Advanced Gastric Cancer (JLSSG0901). World J Surg 2015;39:2734-41. [Crossref] [PubMed]
- Park SH, Sohn TS, Lee J, et al. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. J Clin Oncol 2015;33:3130-6. [Crossref] [PubMed]
- Lee J, Lim DH, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 2012;30:268-73. [Crossref] [PubMed]
- Kang WK, Samsung Medical Center. Phase III Randomized Trial of Adjuvant Chemotherapy With S-1 vs S-1/Oxaliplatin ± Radiotherapy for Completely Resected Gastric Adenocarcinoma: The ARTIST II Trial (ARTIST-II). ClinicalTrials.gov Identifier: NCT01761461. Available online: https://clinicaltrials.gov/ct2/show/NCT01761461
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Ronellenfitsch U, Schwarzbach M, Hofheinz R, et al. Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. Cochrane Database Syst Rev 2013.CD008107. [PubMed]
- Ito S, Ito Y, Misawa K, et al. Neoadjuvant chemotherapy followed by surgery in gastric cancer patients with extensive lymph node metastasis. World J Clin Oncol 2015;6:291-4. [Crossref] [PubMed]
- Inoue K, Nakane Y, Kogire M, et al. Phase II trial of preoperative S-1 plus cisplatin followed by surgery for initially unresectable locally advanced gastric cancer. Eur J Surg Oncol 2012;38:143-9. [Crossref] [PubMed]
- Yoshida K, Yamaguchi K, Okumura N, et al. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer 2016;19:329-38. [Crossref] [PubMed]
- Sato Y, Ohnuma H, Nobuoka T, et al. Conversion therapy for inoperable advanced gastric cancer patients by docetaxel, cisplatin, and S-1 (DCS) chemotherapy: a multi-institutional retrospective study. Gastric Cancer 2017;20:517-26. [Crossref] [PubMed]
- Kinoshita J, Fushida S, Tsukada T, et al. Efficacy of conversion gastrectomy following docetaxel, cisplatin, and S-1 therapy in potentially resectable stage IV gastric cancer. Eur J Surg Oncol 2015;41:1354-60. [Crossref] [PubMed]
- Guner A, Son T, Cho I, et al. Liver-directed treatments for liver metastasis from gastric adenocarcinoma: comparison between liver resection and radiofrequency ablation. Gastric Cancer 2016;19:951-60. [Crossref] [PubMed]
Cite this article as: Guner A. Recent trends of gastric cancer treatment in Turkey. Transl Gastroenterol Hepatol 2017;2:31.


