Lymphadenectomy: how to do it?
Introduction
According to the more recent European guidelines, the D2 lymphadenectomy is considered the standard for curative intent treatment of patients with gastric cancer (1-6). Although, the surgical definition of D2 dissection and its technical aspects had been learned from Eastern surgeons in the past decades, some variations in the approach to D2 lymphadenectomy by European surgeons were detectable in the most important European randomized clinical trials on lymphadenectomy (7,8). To this regard, very recently, the Dutch Gastric Cancer Trial group (9) reported that major non-compliance, defined as the absence of retrieved lymph nodes in the nodal stations included in the intended extent of D2 dissection, occurred in 26% of the D2 procedures. In more recent years, an improvement in quality of lymphadenectomy has been reported in clinical studies and randomized clinical trials (10,11) as a consequence of the more attention paid by dedicated surgeons or due to nationwide audit projects (12), but some differences in the practice of D2 dissection are thought to persist. As, these may contribute to discrepancies in gastric cancer survival observed across European countries (13), the standardization of surgical quality is an urgent need to improve the outcome of gastric cancer patients in Europe (14). The aim of this manuscript is to focus on the technical aspects of the D2 dissection both in open and laparoscopic gastrectomy in order to contribute to the improvement of surgical care of gastric cancer in the West.
Surgical technique—D2 lymphadenectomy
In open gastrectomy with D2 lymphadenectomy, we start by performing a median xifo-umbilical laparotomy then we proceed with the resection of left triangular ligament to mobilize liver segments II and III medially and expose the cardial region.
At this point, we perform a complete detachment of the greater omentum from the transverse mesocolon along the avascular plane. This dissection is continued toward the right and left with mobilization of hepatic and splenic colic flexures. On the left side, during colo-epiploic detachment, we find the left gastroepiploic artery and vein; these are cut at their origin from the splenic vessels allowing the removal of station No. 4sb.
For tumors of the posterior gastric wall penetrating the serosa a bursectomy with complete removal of the inner peritoneal surface of the bursa omentalis is performed.
Then, after opening the lesser omentum, we place a tape around the gastric body, in this way the stomach is pulled-up so that by tractioning caudally the transverse colon, we have a good exposure of the origin of right gastroepiploic vessels.
Hence, we first proceed to isolation and section of the right gastroepiploic vein proximally to its confluence with the middle colic vein, then we perform the section of the right gastroepiploic artery at its origin very close to pancreas border; at this time lymphadenectomy of nodes at station No. 6 (infrapyloric nodes) is performed removing the adipose tissue located between the trunk of Henle and the antero-superior pancreaticoduodenal vein together with dissection of corresponding portion of front face of pancreatic serosa. Of note, in this way infrapyloric nodes (station No. 6) and lymph nodes along the right gastroepiploic artery (station No. 4d) are dissected en bloc.
At this point, we proceed with the dissection of right gastric vessels that is performed at their origin thus removal of suprapyloric nodes (station No. 5) is completed. The pylorus and the duodenal bulb are now mobilized so that we can transect the duodenum with a mechanical linear stapler placed 2–3 cm below the pylorus. Then a complete division of lesser omentum is done.
In case of total gastrectomy, gastric mobilization continues with resection of the gastrosplenic ligament carefully ligating short gastric vessels next to the splenic hilum to ensure correct lymphadenectomy at station No. 4sa. While, if a sub-total gastrectomy is needed, short gastric vessels are obviously preserved, anyway it is important to dissect the gastrosplenic ligament until the first short gastric vessel is met, indeed to ensure a complete dissection of station No. 4sb, the greater curvature needs to be cleared of the omentum starting from the insertion of short gastric arteries to Van Goethem point which is the connection point between the right and the left gastroepiploic arcades.
Now the stomach is tractioned upwards and medially, while the first assistant, with his right hand, raises the gastro-pancreatic fold and with his left hand pushes the pancreatic body in order to allow the isolation and section of left gastric vessels. First, left gastric vein is ligated just above the upper pancreatic margin, then we identify the left gastric artery at the confluence with hepatic and splenic arteries. The removal of adipose tissue surrounding left gastric vein from level of upper pancreatic border to the origin of left gastric artery allows correct dissection of station No. 7 nodes.
Division of the phrenoesophageal membrane (Laimer-Bertelli membrane) leads to the release of cardia and anterior surface of abdominal esophagus, then, we carefully detach the posterior esophageal wall by ligating the vascular cardial branches and perform the section of the two vagus nerves. In this way we obtain an optimal mobilization of the esophagus allowing a complete en bloc removal of right para-cardial nodes and of nodes along the first branch of left gastric artery (station No. 1). If a total gastrectomy is performed, the opening of Laimer-Bertelli membrane along the left diaphragmatic pillar, is necessary to perform the en bloc dissection of left paracardial nodes (station No. 2), while in case of sub-total gastrectomy, the Laimer-Bertelli membrane should be not completely opened, it is enough to dissect its right side up to the right esophageal wall.
When performing laparoscopic gastrectomy, after the Laimer-Bertelli membrane is opened, to avoid esophageal wall injuries, it is better to start the dissection from the lesser curvature: once the gastric wall is identified, then we move up-ward along the cardia and esophageal wall, thus making an en bloc resection of nodes at stations No. 1 and No. 3. At this point a D1 lymphadenectomy is made.
To complete a D2 lymphadenectomy, prior to reconstruction, node dissection along the common hepatic artery, the celiac trunk and the splenic artery is performed.
The celiac trunk should be skeletonized in the tract included among the origin of the common hepatic artery and the splenic artery: in this way, all the soft tissue surrounding the celiac trunk is detached from the retroperitoneum (station No. 9).
The next step implies the section of the posterior parietal peritoneum along the superior margin of the pancreas in order to skeletonize the common hepatic artery until the origin of the gastroduodenal trunk, indeed, the removal of the fat tissue included between the common hepatic artery, the gastroduodenal trunk and the anterior surface of pancreatic head allow the complete dissection of nodal station No. 8a. The splenic artery is skeletonized leftwards in its proximal tract until the origin of the posterior gastric artery (station No. 11p) and around the splenic artery to the tip of the pancreas tail (station No. 11d).
Extending right-wards the dissection of the proper hepatic artery the hepatoduodenal ligament is opened and the anterior lymph nodes of the hepato-duodenal ligament are also removed (station No. 12a), this dissection can be considered complete if it is conducted until the portal vein becomes visible.
The line of dissection follows the perivascular autonomic nerve branches to avoid devascularization of the structures particularly the main bile duct. In case of evident or suspected nodal involvement of the posterior hepato-duodenal region, lymphadenectomy of this region has to be carried out, but in these cases, particular care should be held on preserving the vascularization of the bile duct and accidental portal injuries. To this regard, it can be useful to push upward the portal vein by inserting a finger trough the Winslow foramen.
In case of macroscopic metastasis at splenic hilum it is possible to perform a spleen preserving dissection of lymph nodes at No. 10 station by Jinnai’s maneuver.
First step is section of posterior parietal peritoneum from right to left starting to the left margin of upper mesenteric vein along the lower margin of the pancreas and posterior margin of the spleen up to superior splenic pole. Then, after a careful detachment from posterior structures, the spleno-pancreatic block is turned medially and the dissection of lymph nodes along the splenic artery and at splenic hilum can be performed.
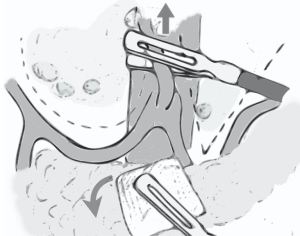
At variance with open procedure, laparoscopic D2 lymphadenectomy is performed en bloc: the dissection starts from the section of left gastric artery, which is pulled upward by the assistant who is at the same time, with the other hand, rolling down the pancreas with the aid of a gauze. In this way, the hepatic and splenic arteries are well exposed and, once the perivascular autonomic plane is found, all the soft tissue on the left and the right sides of the left gastric artery can be removed by imagining a “U” shape dissection line on the right, above the hepatic artery, and a “V” shape dissection line on the left, along the splenic artery (Figure 1). Moreover, when performing the dissection of station 12a, it is possible pressing downward the hepatic artery by grasping the perivascular autonomic layer, in this way the exposure of portal vein is simplified.
Additional notes
With regard to the use of specific devices to perform lymphadenectomy, there is the hypothesis that during gastrectomy for gastric cancer, an improper closure of lymphovascular vessels may cause a spillage of cancer cells into the peritoneal cavity. Specifically, Yang et al. (15), by conducting ex vivo experiments on surgical specimens, demonstrated that if lymphovascular channels are not properly sealed, free cancer cells are spilled out. Therefore, during lymphadenectomy for gastric cancer, dissection with scissors or scalpel which may cause the opening of vessels, should be avoided, moreover all the visible lymphovascular vessels should be carefully closed with the use of clips or though energy-based devices (15).
As for the handling of surgical specimen after D2 lymphadenectomy, it is known that immediate dissection and collection of lymph nodes by separate stations on the fresh specimen is routinely performed by Japanese and Korean surgeons in order to improve the nodal retrieval by the pathologists. This has been recently confirmed also in two western studies (16,17) in which ex vivo nodal dissection was associated with an increased lymph node yield. Therefore, lymph node pick up by separate stations should be considered also in the West to provide a proper pathological staging and thus a more precise prognostic prediction after surgery for gastric cancer (16,17).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Meyer HJ, Hölscher AH, Lordick F, et al. Current S3 guidelines on surgical treatment of gastric carcinoma. Chirurg 2012;83:31-7. [Crossref] [PubMed]
- Allum WH, Blazeby JM, Griffin SM, et al. Guidelines for the management of oesophageal and gastric cancer. Gut 2011;60:1449-72. [Crossref] [PubMed]
- Okines A, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21:v50-v54. [Crossref] [PubMed]
- Waddell T, Verheij M, Allum W, et al. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol. 2014;40:584-91. [Crossref] [PubMed]
- De Manzoni G, Marrelli D, Baiocchi GL, et al. The Italian Research Group for Gastric Cancer (GIRCG) guidelines for gastric cancer staging and treatment: 2015. Gastric Cancer 2017;20:20-30. [Crossref] [PubMed]
- Verlato G, Giacopuzzi S, Bencivenga M, et al. Problems faced by evidence-based medicine in evaluating lymphadenectomy for gastric cancer. World J Gastroenterol 2014;20:12883-91. [Crossref] [PubMed]
- Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 1999;79:1522-30. [Crossref] [PubMed]
- Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999;340:908-14. [Crossref] [PubMed]
- de Steur WO, Hartgrink HH, Dikken JL, et al. Quality control of lymph node dissection in the Dutch Gastric Cancer Trial. Br J Surg 2015;102:1388-93. [Crossref] [PubMed]
- Marrelli D, Pedrazzani C, Morgagni P, et al. Changing clinical and pathological features of gastric cancer over time. Br J Surg 2011;98:1273-83. [Crossref] [PubMed]
- Degiuli M, Sasako M, Ponti A, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg 2014;101:23-31. [Crossref] [PubMed]
- Busweiler LA, Wijnhoven BP, van Berge Henegouwen MI, et al. Early outcomes from the Dutch Upper Gastrointestinal Cancer Audit. Br J Surg 2016;103:1855-63. [Crossref] [PubMed]
- Dikken JL, van Sandick JW, Allum WH, et al. Differences in outcomes of oesophageal and gastric cancer surgery across Europe. Br J Surg 2013;100:83-94. [Crossref] [PubMed]
- Verlato G, Roviello F, Marchet A, et al. Indexes of surgical quality in gastric cancer surgery: experience of an Italian network. Ann Surg Oncol 2009;16:594-602. [Crossref] [PubMed]
- Han TS, Kong SH, Lee HJ, et al. Dissemination of free cancer cells from the gastric lumen and from perigastric lymphovascular pedicles during radical gastric cancer surgery. Ann Surg Oncol 2011;18:2818-25. [Crossref] [PubMed]
- Morgagni P, Nanni O, Carretta E, et al. Lymph node pick up by separate stations: Option or necessity. World J Gastrointest Surg 2015;7:71-7. [Crossref] [PubMed]
- Afaneh C, Levy A, Selby L, et al. Ex Vivo Lymphadenectomy During Gastrectomy for Adenocarcinoma Optimizes Lymph Node Yield. J Gastrointest Surg 2016;20:165-71; discussion 171. [Crossref] [PubMed]
Cite this article as: Giacopuzzi S, Bencivenga M, Cipollari C, Weindelmayer J, de Manzoni G. Lymphadenectomy: how to do it? Transl Gastroenterol Hepatol 2017;2:28.


