Prognostic impact of nodal status and therapeutic implications
Introduction
Surgical resection remains the cornerstone for cure in the treatment of gastric cancer. Lymphatic spread is one of the most important prognostic factors in gastric cancer beside complete tumor resection. A D2 lymphadenectomy (LAD) is nowadays the accepted surgical standard in western centers (1-3). A number of at least 25 lymph nodes (LNs) are suggested in the German S3 guidelines for the surgical treatment of gastric cancer as an adequate number of LNs for a D2 lymphadenectomy. Individual adjuvant treatment decisions mostly depend on resection category and nodal status (4,5). It is now generally accepted that not only the presence of LN metastases but also the number of metastatic LNs might be a predictor of survival (6-10). The number of LNs required to be examined for accurate staging is 16 in gastric cancer and lies under the required number of LNs for a D2 LAD (11,12). This is a particular problematic issue since nodal staging according to the number of positive LNs is influenced by the extent of LAD. A greater extent of LAD is associated with improved survival (6-10,13). Also, the lymph node ratio (LNR) has been described as independent predictor of survival by several authors (13-20). But the LNR does not represent a generally established prognostic factor and has not been integrated into the current staging system. Furthermore, there is no standardized definition and classification with regard to the LNR, although many authors use 0.2 as a cut off value, achieving marked prognostic relevance (14). The role of lymphatic spread as a relevant prognostic factor in neoadjuvantly treated patients is still under investigation. The UICC staging system is only based on primarily resected patients, but the ypN-category and LNR seem to play an important prognostic role. Very limited data exist for the prognostic influence of response to chemotherapy of the involved LNs (21). Furthermore there might be an association of the response of the primary tumor and ypN-category (Table 1) (22). The better the response of the primary tumor, the lower the ypN-category (Table 1). The extent of LAD starts to be discussed again due to the application of more aggressive chemotherapy with high response rates of the primary tumor, the increasing rate of laparoscopic approaches and the treatment of older patients with comorbidities (23-25). Therefore, again a more limited LN dissection is discussed (24).

Full table
Research criteria
Prognostic role in primarily resected gastric cancer
While the immense prognostic influence of nodal involvement in gastric cancer is generally accepted, the extent of LAD is still controversially discussed (6-10,13). Several recent publications have reported a survival benefit after extended LN dissection, but the question whether improved survival is due to a more accurate staging or caused by a genuine therapeutic effect of extensive LAD remains unanswered. Furthermore, not all patients seem to benefit equally from an extensive LAD, it seems to depend on nodal involvement and/or depth of tumor invasion. Moreover, several prognostic factors have been identified—e.g., total number of LNs removed irrespective of tumor involvement, number of negative LNs, number of positive LNs, and LNR (6-11,13-20). The suggested range is large and all data are based on retrospective, often multicenter analyses, since no prospective randomized trials are available. Thus, the debate on the necessary number of LNs is ongoing. Even for patients classified as pN0 recommendations for an adequate LAD range between 15–26 harvested LNs for correct classification and prognostic impact (11,12,26-28). For oncological reasons, we suggest, that all patients, who have the chance of a R0 resection, should have a D2 LAD best with at least 25 removed LNs according to the German S3 guidelines, not only for accurate pathological staging, but also for potential improvement of survival, irrespective of the later pN-category. Additionally only a LAD with far more LN removed than involved, guarantees a real R0 resection, otherwise it might be possible that positive nodes remain in situ and the resection might be a R2 resection in fact. We suggest that the surgeon should perform a meticulous LAD to potentially decrease the LNR by increasing the total LN count. The LAD besides a complete resection is the only oncological factor that can be influenced by the surgeon. In many reports the LNR is judged to improve the TNM staging classification and reduce the stage migration of UICC N-categories (10,18,19). Further the LNR is associated with adverse pathological features like advanced T-stage, lymphangiosis, vascular or perineural invasion and is a negative prognostic factor (14,17). The grouping of LNR with prognostic impact varies from two groups ≤20% (14), ≤40% for the subgroup of pN3 (15), up to four groups 0 vs. 1–9% vs. 10–25% vs. >25% (19,20,29) or 0 vs. 1–30% vs. 31–60% vs. <60% (17). The best prognostic cut-off remains unclear, however often groups of 0%, 1–20% and 20% are used in clinical routine with a good prediction of prognosis.
Apart from the number of removed and involved LNs and the resulting pN-category and LNR, biological factors like lymphovascular invasion are of prognostic relevance. Several studies have shown the negative prognostic impact of lymphangiosis in all resected gastric cancer patients (30,31) or even in pN0 (32,33) and suggested adjuvant therapies in this subgroup of patients (30,32).
An additional poor prognosticator is micro metastases in LNs. Micro metastases could be identified as risk factors for recurrent gastric cancer and poorer survival (34-36). The percentage of micro metastases ranges from 1.8% in pT1a gastric cancer (37) up to 32% (34,36). Micro metastases were observed beyond the perigastric nodes along the left gastric or common hepatic artery, which underlies the importance of a D2 LAD for gastric cancer (34).
Many have doubted the benefit of an extended LAD in spite of its potentially positive influence on survival, because in the past it was assumed to increase postoperative morbidity and mortality (38). But several studies have shown low morbidity and mortality rates (1-3,23). Recently this aspect was also proven for laparoscopic D2 LAD in a randomized trial (24).
Prognostic role of LN metastases in neoadjuvantly treated gastric cancer
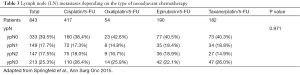
Most studies aiming to evaluate prognostic role of node metastasis excluded patients who received neoadjuvant treatment, even though nowadays the percentage of preoperatively treated patients with gastric cancer is steadily increasing (39-41). In all three randomized trials on neoadjuvant treatment the numbers of LNs removed and involved are not reported in the respective study arms. Only one retrospective study with a very limited patient number (58 neoadjuvantly treated patients versus 168 patients with surgery alone) addresses the aspect of number of removed LNs. Of note an association of a retrieval of less than 15 LNs after the application of chemotherapy was reported (24.1% vs. 7.7% after primary resection) (42), which does not correspond at all to our clinical experience after D2 LAD in locally advanced gastric cancer (see below). Also, the LNR and lymphangiosis were not addressed in the three RCTs mentioned above. Whereas the MAGIC and FFCD study did not show a significant increase of ypN0 patients after chemotherapy, in the EORTC 40954 study the rate of ypN0 patients was significantly increased after preoperative chemotherapy with 39% compared to merely 19% after primary surgery (Table 2) (40). The ypN-category was confirmed in large series of neoadjuvantly treated gastric cancer patients as an independent prognostic factor (22,25) correspondingly to primarily resected gastric cancer. Neither the type of chemotherapy, nor double or triple chemotherapy nor the application of taxanes did influence significantly the ypN-categories (Table 3) (25). In contrast to a recently started discussion, our data do not justify to reduce the LAD from a D2 to a D1 LAD in preoperatively with taxanes treated patients due to higher ypN0 rates. However, a strong association of the response of the primary tumor and LN metastases could be shown in a large retrospective analysis. Whereas 79% of patients with a complete pathological response after chemotherapy are classified as ypN0, at least 58% of the patients with less than 10% residual tumor are ypN0 compared to merely 25% with more than 50% of the residual primary tumor (Table 1) (22).

Full table
Full table
Prognostic role of LN response in neoadjuvantly treated gastric cancer
Histopathological response is regarded as an important prognostic factor in patients undergoing neoadjuvant therapy for locally advanced gastric cancer (22,25,43). Until now it is unknown whether the prognostic impact is the same for response to chemotherapy of the primary tumor and the response of LN metastases. The histopathological work up for the scientific question is demanding. One paper is addressing this topic (21) to our knowledge. Twenty-eight patients with 438 metastatic LNs were examined. Five percent of the LNs showed a pathological complete response, 11% had less than 10% residual tumor, 14% had 11–50% residual tumor and 70% had more than 50% residual tumor. They concluded that preoperative chemotherapy did not provide any outstanding histopathological benefit for metastatic LNs with the conclusion that an appropriate D2 LAD is required to cure the patients (21). Unpublished data from Munich confirm this finding.
From 1987–2007, 686 patients with locally advanced gastroesophageal cancer were resected after they had been treated preoperatively with platinum-5-FU-based chemotherapy. A regression of less than 10% residual tumor cells in the primary tumor or LN specimen was defined as histopathological response (Figure 1). All sampled LNs (mean: n=31 per patient) were examined for histopathological regression.
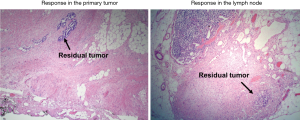
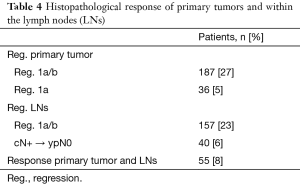
Histopathological response was seen in 187 (27.3%) of the primary tumors, including 36 (5.2%) complete responses. In 157 patients (22.9%) LN metastases responded, but only 40 (5.8%) patients were down staged from cN+ to ypN0, which corresponds perfect with the Japanese data shown above. Combined response of the primary tumor and LNs was evident in merely 55 patients (8.0%) (Table 4). Response of the primary tumor was correlated with a favorable prognosis (P<0.001), but response in the LNs alone was not (P=0.32) (Figure 2). Response of the primary tumor was associated with grading (P=0.001), intestinal subtype (P=0.002), lower ypT-, ypN-categories and less lymphangiosis carcinomatosa (all P<0.001). Response of the primary tumor had particular prognostic importance within the ypT2- (P=0.003) and ypN1- category (P=0.002).
Full table
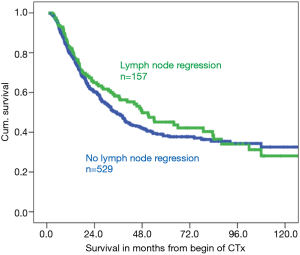
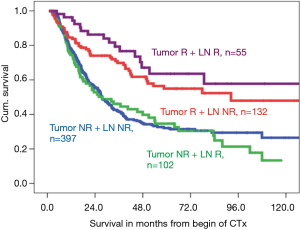
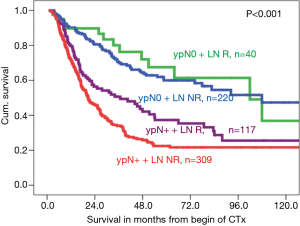
Patients with regression in both the primary tumor and LNs had the best prognosis, followed by patients with regression of the primary tumor and no LN regression, whereas the prognostic influence of LN regression in patients with nonresponse of the primary tumor is less or even not relevant for prognosis (Figure 3). Within nodal negative patients LN response seems less relevant for prognosis, than in nodal positive patients, in whom lymphatic regression seems to increase prognosis (Figure 4).
Our unpublished data suggests that responses in the primary tumor but not in the metastatic LNs are of primary prognostic relevance. These observations merit validation in other patient cohorts. Future clinical studies should focus on the value of adjuvant treatment in specific subgroups.
Therapeutic implications of LAD and nodal status
To guarantee an accurate pathological staging and to minimize stage migration an adequate D2 LAD in gastric cancer with at least 25 resected LNs as measurable criterion according to the German S3 guidelines should be performed.
In Europe a postoperative adjuvant chemotherapy identical to the preoperative therapy scheme is standard in preoperatively treated patients, irrespective of the nodal status or response to the preoperative chemotherapy (39,41).
Patients with primary surgery with postoperative risk factors like insufficient LAD, a high LNR, lymphangiosis carcinomatosa or micro metastases might be discussed for further individual adjuvant treatment (30,32). Whether adjuvant chemotherapy or chemoradiotherapy should be delivered in these patients remains unclear so far. However in nodal positive patients or patient with lymphatic risk factors chemoradiotherapy might be preferred due to the data of the ARTIST trial (44).
Generally, the benefit of adjuvant chemoradiotherapy after D2 LAD remains unclear. A recent meta-analysis suggests that a chemoradiotherapy after D2 LAD might be associated with longer 5-year overall survival, but might not improve 5-year disease free survival (5). A further meta-analysis comparing postoperative chemoradiotherapy and postoperative chemotherapy in primarily resected patients with D2 LAD in a non-selected Asian population shows, that chemoradiotherapy improves locoregional recurrence free survival, but not overall survival compared to a chemotherapy alone (45). A recently published randomized trial for D2 resected gastric cancer patients (ARTIST Trial) showed that both adjuvant chemotherapy and chemoradiotherapy are tolerated and equally beneficial in preventing relapse. A subgroup analysis showed that chemoradiotherapy significantly improved disease free survival in nodal positive patients and in patients with intestinal Lauren subtype (44).
A propensity score matched analysis comparing the efficacy of adjuvant chemoradiotherapy and chemotherapy alone was performed on 3,008 resected gastric cancer patients in the US. Chemoradiotherapy showed a better overall survival compared to chemotherapy alone regardless of stage. This effect was pronounced in patients with inadequate LAD and less pronounced with increasing number of resected LNs. However chemoradiotherapy improves overall survival in patients with LN metastases irrespective of the adequacy of LAD. In node negative patients, only the patients with inadequate LAD had a benefit from chemoradiotherapy compared to chemotherapy alone (4). Based on these data LN status and quality of LAD might influence adjuvant therapy selection in the United States.
Conclusions
LN metastases remain one of the most relevant prognostic factors in primary resected gastric cancer. Also, the number of resected nodes-involved or not involved, LN ratio micrometastases and lymphangiosis are reported to be relevant prognostic factors. The only factor which can positively influenced by the surgeon beside an R0 resection is the quality of lymphadenectomy. Actually, a D2 LAD should be recommended for patients with a possible R0 resection to improve prognosis. The number of LNs required for a D2 resection is still discussed controversially, but the German S3 guidelines recommend at least 25 removed LNs based on former anatomically findings as a criterion for a D2 resection.
In neoadjuvantly treated patients the ypN category remains a strong prognosticator since the TNM classification is based on primary resected patients only. Only one of the three randomized studies shows an increase of the ypN0 category by preoperative treatment. Also, none of the established applicated preoperative chemotherapy regimens seems to increase the ypN0 category significantly. So, it might not generally be assumed that any chemotherapy might increase the number of patients with ypN0 category. Furthermore, there are no published data so far, which allow reducing a D2 to a D1 LAD after preoperative treatment. The response of the primary tumor seems to be more important for survival than the response of the LNs.
In Europe, perioperative chemotherapy is standard irrespectively of type of lymphadenectomy, LN metastases or response of the primary tumor on preoperative therapy. However, there are limited data mostly from the US that patients with LN metastases and an inadequate LAD might profit from an adjuvant chemoradiotherapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [Crossref] [PubMed]
- de Steur WO, Hartgrink HH, Dikken JL, et al. Quality control of lymph node dissection in the Dutch Gastric Cancer Trial. Br J Surg 2015;102:1388-93. [Crossref] [PubMed]
- Mocellin S, McCulloch P, Kazi H, et al. Extent of lymph node dissection for adenocarcinoma of the stomach. Cochrane Database Syst Rev 2015.CD001964. [PubMed]
- Datta J, McMillan MT, Ecker BL, et al. Implications of Lymph Node Staging on Selection of Adjuvant Therapy for Gastric Cancer in the United States: A Propensity Score-matched Analysis. Ann Surg 2016;263:298-305. [Crossref] [PubMed]
- Liang JW, Zheng ZC, Yu T, et al. Is postoperative adjuvant chemoradiotherapy efficacious and safe for gastric cancer patients with D2 lymphadenectomy? A meta-analysis of the literature. Eur J Surg Oncol 2014;40:1614-21. [Crossref] [PubMed]
- Okajima W, Komatsu S, Ichikawa D, et al. Prognostic impact of the number of retrieved lymph nodes in patients with gastric cancer. J Gastroenterol Hepatol 2016;31:1566-71. [Crossref] [PubMed]
- Deng J, Yamashita H, Seto Y, et al. Increasing the Number of Examined Lymph Nodes is a Prerequisite for Improvement in the Accurate Evaluation of Overall Survival of Node-Negative Gastric Cancer Patients. Ann Surg Oncol 2017;24:745-53. [Crossref] [PubMed]
- Lu J, Wang W, Zheng CH, et al. Influence of Total Lymph Node Count on Staging and Survival After Gastrectomy for Gastric Cancer: An Analysis From a Two-Institution Database in China. Ann Surg Oncol 2017;24:486-93. [Crossref] [PubMed]
- Gholami S, Janson L, Worhunsky DJ, et al. Number of Lymph Nodes Removed and Survival after Gastric Cancer Resection: An Analysis from the US Gastric Cancer Collaborative. J Am Coll Surg 2015;221:291-9. [Crossref] [PubMed]
- Chen HN, Chen XZ, Zhang WH, et al. Necessity of harvesting at least 25 lymph nodes in patients with stage N2-N3 resectable gastric cancer: a 10-year, single-institution cohort study. Medicine (Baltimore) 2015;94:e620. [Crossref] [PubMed]
- Biondi A, D'Ugo D, Cananzi FC, et al. Does a minimum number of 16 retrieved nodes affect survival in curatively resected gastric cancer? Eur J Surg Oncol 2015;41:779-86. [Crossref] [PubMed]
- Son T, Hyung WJ, Lee JH, et al. Clinical implication of an insufficient number of examined lymph nodes after curative resection for gastric cancer. Cancer 2012;118:4687-93. [Crossref] [PubMed]
- Chen S, Zhao BW, Li YF, et al. The prognostic value of harvested lymph nodes and the metastatic lymph node ratio for gastric cancer patients: results of a study of 1,101 patients. PloS One 2012;7:e49424. [Crossref] [PubMed]
- Komatsu S, Ichikawa D, Nishimura M, et al. Evaluation of prognostic value and stage migration effect using positive lymph node ratio in gastric cancer. Eur J Surg Oncol 2017;43:203-9. [Crossref] [PubMed]
- Komatsu S, Ichikawa D, Miyamae M, et al. Positive Lymph Node Ratio as an Indicator of Prognosis and Local Tumor Clearance in N3 Gastric Cancer. J Gastrointest Surg 2016;20:1565-71. [Crossref] [PubMed]
- Wu XJ, Miao RL, Li ZY, et al. Prognostic value of metastatic lymph node ratio as an additional tool to the TNM stage system in gastric cancer. Eur J Surg Oncol 2015;41:927-33. [Crossref] [PubMed]
- Melis M, Masi A, Pinna A, et al. Does lymph node ratio affect prognosis in gastroesophageal cancer? Am J Surg 2015;210:443-50. [Crossref] [PubMed]
- Nelen SD, van Steenbergen LN, Dassen AE, et al. The lymph node ratio as a prognostic factor for gastric cancer. Acta Oncol 2013;52:1751-9. [Crossref] [PubMed]
- Alatengbaolide Lin D. Lymph node ratio is an independent prognostic factor in gastric cancer after curative resection (R0) regardless of the examined number of lymph nodes. Am J Clin Oncol 2013;36:325-30. [Crossref] [PubMed]
- Marchet A, Mocellin S, Ambrosi A, et al. The prognostic value of N-ratio in patients with gastric cancer: validation in a large, multicenter series. Eur J Surg Oncol 2008;34:159-65. [Crossref] [PubMed]
- Kinoshita O, Ichikawa D, Ichijo Y, et al. Histological evaluation for chemotherapeutic responses of metastatic lymph nodes in gastric cancer. World J Gastroenterol 2015;21:13500-6. [Crossref] [PubMed]
- Schmidt T, Sicic L, Blank S, et al. Prognostic value of histopathological regression in 850 neoadjuvantly treated oesophagogastric adenocarcinomas. Br J Cancer 2014;110:1712-20. [Crossref] [PubMed]
- Kawaguchi T, Komatsu S, Ichikawa D, et al. Prognostic Influence of the Extent of Lymph Node Dissection and Perioperative Comorbidities in Patients with Gastric Cancer. Anticancer Res 2016;36:1917-22. [PubMed]
- Hu Y, Huang C, Sun Y, et al. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol 2016;34:1350-7. [Crossref] [PubMed]
- Springfeld C, Wiecha C, Kunzmann R, et al. Influence of Different Neoadjuvant Chemotherapy Regimens on Response, Prognosis, and Complication Rate in Patients with Esophagogastric Adenocarcinoma. Ann Surg Oncol 2015;22 Suppl 3:S905-14. [Crossref] [PubMed]
- Chu X, Yang ZF. Impact on survival of the number of lymph nodes resected in patients with lymph node-negative gastric cancer. World J Surg Oncol 2015;13:192. [Crossref] [PubMed]
- He H, Shen Z, Wang X, et al. Survival benefit of greater number of lymph nodes dissection for advanced node-negative gastric cancer patients following radical gastrectomy. Jpn J Clin Oncol 2016;46:63-70. [Crossref] [PubMed]
- Martinez-Ramos D, Calero A, Escrig-Sos J, et al. Prognosis for gastric carcinomas with an insufficient number of examined negative lymph nodes. Eur J Surg Oncol 2014;40:358-65. [Crossref] [PubMed]
- Marchet A, Mocellin S, Ambrosi A, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg 2007;245:543-52. [Crossref] [PubMed]
- Lagarde SM, Phillips AW, Navidi M, et al. The presence of lymphovascular and perineural infiltration after neoadjuvant therapy and oesophagectomy identifies patients at high risk for recurrence. Br J Cancer 2015;113:1427-33. [Crossref] [PubMed]
- Li P, He HQ, Zhu CM, et al. The prognostic significance of lymphovascular invasion in patients with resectable gastric cancer: a large retrospective study from Southern China. BMC Cancer 2015;15:370. [Crossref] [PubMed]
- Lee JH, Kim MG, Jung MS, et al. Prognostic significance of lymphovascular invasion in node-negative gastric cancer. World J Surg 2015;39:732-9. [Crossref] [PubMed]
- Du CY, Chen JG, Zhou Y, et al. Impact of lymphatic and/or blood vessel invasion in stage II gastric cancer. World J Gastroenterol 2012;18:3610-6. [Crossref] [PubMed]
- Lee CM, Cho JM, Jang YJ, et al. Should lymph node micrometastasis be considered in node staging for gastric cancer?: the significance of lymph node micrometastasis in gastric cancer. Ann Surg Oncol 2015;22:765-71. [Crossref] [PubMed]
- Zeng YJ, Zhang CD, Dai DQ. Impact of lymph node micrometastasis on gastric carcinoma prognosis: a meta-analysis. World J Gastroenterol 2015;21:1628-35. [Crossref] [PubMed]
- Yasuda K, Adachi Y, Shiraishi N, et al. Prognostic effect of lymph node micrometastasis in patients with histologically node-negative gastric cancer. Ann Surg Oncol 2002;9:771-4. [Crossref] [PubMed]
- Lee T, Tanaka H, Ohira M, et al. Clinical impact of the extent of lymph node micrometastasis in undifferentiated-type early gastric cancer. Oncology 2014;86:244-52. [Crossref] [PubMed]
- Memon MA, Subramanya MS, Khan S, et al. Meta-analysis of D1 versus D2 gastrectomy for gastric adenocarcinoma. Ann Surg 2011;253:900-11. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol 2010;28:5210-8. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Wu ZM, Teng RY, Shen JG, et al. Reduced lymph node harvest after neoadjuvant chemotherapy in gastric cancer. J Int Med Res 2011;39:2086-95. [Crossref] [PubMed]
- Becker K, Langer R, Reim D, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg 2011;253:934-9. [Crossref] [PubMed]
- Park SH, Sohn TS, Lee J, et al. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. J Clin Oncol 2015;33:3130-6. [Crossref] [PubMed]
- Huang YY, Yang Q, Zhou SW, et al. Postoperative chemoradiotherapy versus postoperative chemotherapy for completely resected gastric cancer with D2 Lymphadenectomy: a meta-analysis. PloS One 2013;8:e68939. [Crossref] [PubMed]
Cite this article as: Ott K, Blank S, Ruspi L, Bauer M, Sisic L, Schmidt T. Prognostic impact of nodal status and therapeutic implications. Transl Gastroenterol Hepatol 2017;2:15.


