Regorafenib: lights and shadows of antiangiogenic therapies in gastric cancer
Gastric cancer stands among the most common causes of cancer death worldwide (1). Median overall survival (OS) for patients with advanced disease is approximately 1 year and chemotherapy still represents the milestone of treatment in most patients (2). While first-line chemotherapy has a well-established role in gastric cancer management, during the past few years several trials clearly demonstrated a significant OS benefit for second-line therapy compared with best supportive care (BSC) alone (3); however, as the gain in survival is at best modest in absolute terms, a careful clinical selection is needed to identify patients who could benefit from salvage therapies (4). Moreover, no standard treatment exists after second-line failure. Thus, searching for new therapeutic opportunities in pretreated advanced gastric cancer patients is imperative for researchers and clinicians.
Different antiangiogenic agents have been tested with mixed results (Table 1) (5-14). While monoclonal antibodies have to date failed to improve outcome in the first-line setting (5,6,9), ramucirumab [an antibody against the vascular endothelial growth factor receptor 2 (VEGFR2)] has been the first targeted therapy that significantly increased OS in second-line, either alone compared with placebo (7) or in combination with paclitaxel (TXL) compared with single agent TXL (8). Therefore, in spite of some relevant failures of the antiangiogenic approach in this disease, VEGFR2 confirmed to be a useful target for new cancer therapies. Indeed, different tyrosine kinase inhibitors (TKIs) have been evaluated in pretreated patients with promising results. In particular, apatinib, an oral anti-VEGFR2 TKI, significantly improved OS and progression-free survival (PFS) compared with placebo in patients with advanced gastric cancer refractory to two or more lines of prior chemotherapy (10,11).
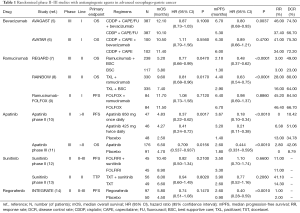
Full table
In this regard, the recently reported data with regorafenib are of interest. Pavlakis et al. published the results of a randomized, double-blind, phase 2 trial (INTEGRATE) evaluating regorafenib in patients with advanced oesophago-gastric junction cancer or gastric cancer, refractory to two or fewer lines of chemotherapy (including platinum and fluoropyrimidines) (14). A total of 152 patients were enrolled between November 2012 and February 2014 and randomly assigned to either regorafenib (at the dose of 160 mg daily from day 1 to 21 of each 28-day cycle) or placebo, both combined with BSC. Patients received active treatment or matching placebo until disease progression or unacceptable toxicity. Among the 147 evaluable patients, median PFS (the primary endpoint of the trial) was 2.6 and 0.9 months in the regorafenib and placebo groups, respectively (HR 0.40; 95% CI: 0.28–0.59; P<0.001). A trend towards improved OS with regorafenib was also reported (median: 5.8 vs. 4.5 months; HR 0.74; 95% CI: 0.51–1.08; P=0.147). As expected, objective responses were sporadic in the regorafenib arm, with only three patients experiencing complete or partial responses according to RECIST version 1.1. The safety profile of the TKI was similar to that expected from previous trials and 32% of the patients receiving regorafenib experienced at least one serious adverse event: the most common severe (grade 3–5) toxicities included transaminases level increase (17%), gastrointestinal disorders (11%), hypertension (10%) and skin toxicity (6%).
Demonstrating signals of efficacy in a difficult patient population is certainly an intriguing finding, and randomized phase II trials with a comparative arm representing standard practice control offer good opportunities to pick new candidates for further clinical evaluation and preliminary investigate potential predictive biomarkers. INTEGRATE thus confirms that angiogenesis is a valuable target for gastric cancer treatment (at least in later lines of therapy, and at least for VEGFR2), but also that other molecular pathways may be effectively targeted with TKIs. In fact, regorafenib is a multitargeted agent against not only neoangiogenesis mediators (i.e., VEGFR1, VEGFR2 and TIE-2), but also tumour microenvironment [i.e., platelet-derived growth factor receptor beta, (PDGFbeta)] and oncogenic tyrosine kinase receptors (i.e., RAF, RET and KIT).
Looking at the data, are then only lights on the horizon? To answer this question, first consider the population enrolled in the INTEGRATE trial. Almost half of the patients (42% in both arms) had received only one line of systemic therapy for advanced disease and had an optimal performance status (PS): is placebo an acceptable control arm in second-line for patients with good PS still able to receive salvage chemotherapy (with or without ramucirumab) or ramucirumab alone? On the basis of the current evidence, the trial should have focused on patients progressing after at least two lines of treatment, as performed in the apatinib studies (10,11). If we consider post-progression treatments, we observed an unbalance in favour of the placebo arm: an almost twice as high number of patients (66.7% vs. 35.5%) received active agents after progression in the control arm, and in many cases also doublet or triplet combination chemotherapy was administered (indirectly confirming that many enrolled patients still retained good PS at the end of study treatment). In light of these considerations, are the results really useful to predict the potential impact of regorafenib in the future management of oesophago-gastric cancer? The answer is probably no, as the apparently brightening lights of PFS and OS results are partly clouded by pitfalls in patient selection criteria. We probably need to wait for the final results of the INTEGRATE II trial, the confirmatory phase III study with OS as primary endpoint comparing regorafenib with placebo, which will enrol patients in the third-line setting only (i.e., refractory or intolerant to two lines of prior therapies for recurrent or metastatic disease, comprising at least one platinum and one fluoropyrimidine analogue) (15).
Secondly, even though generally balanced, patient characteristics were not completely super imposable between arms: liver and peritoneal metastases, more than two sites of disease and higher neutrophil-to-lymphocyte ratio (features generally associated with poorer prognosis in advanced gastric cancer) were all numerically more represented in the placebo arm. Due to the low number of patients in a phase II trial, this could have partly impacted on the findings.
Moreover, as the benefit of regorafenib is limited (according to median PFS data) and restricted to a subgroup of patients (as shown by survival curves), the toxicity profile of the TKI is a key issue in an exclusively palliative setting such as pretreated oesophago-gastric cancer. Treatment is certainly feasible, but active surveillance and patient education for early recognition of drug-related adverse events is needed to promptly manage the most common side effects (16). In this regard, future details about quality of life data (which will be separately reported) could add further insights into INTEGRATE efficacy results.
Interestingly, none of the investigated parameters (age, neutrophil-to-lymphocyte ratio, primary tumour location, number of previous lines of therapy, presence of peritoneal metastases, number of sites of disease and baseline plasma vascular endothelial growth factor-A levels) was associated with differential treatment effect in terms of PFS. The only exception was geographic region: drug efficacy does not seem to be associated with ethnicity, but patients from South Korea experienced greater benefit compared to those coming from Australia, New Zealand or Canada (region-by-treatment interaction P<0.001). This is exactly the opposite of what observed in AVAGAST, where OS benefit from bevacizumab was greater in Western countries (5). This observation remains currently unexplained, as well as the potential reasons behind that (beyond obvious differences in drug structure and pharmacodynamics).
Finally, none of the enrolled patients had received prior ramucirumab treatment. If definitively proved effective in the disease, it could be of interest to know if regorafenib could overcome resistance to ramucirumab thanks to its wider mechanism of action. Lessons learned from colorectal cancer with regorafenib and bevacizumab raise some hope (17): however, as we lack reliable biomarkers of efficacy for antiangiogenic treatments (comprising regorafenib), at present it is difficult to anticipate how promising could be a multitargeted TKI (as well as a selective anti-VEGFR2 TKI such as apatinib) after ramucirumab (18).
In conclusion, INTEGRATE is an interesting trial, which prompts some optimism about the future of oesophago-gastric cancer management. While providing lights of expectation, however, it contemporarily throws shadows about the optimal setting for regorafenib administration, the possibility of patient selection beyond clinical criteria, the potential combination with chemotherapy as a maintenance strategy in previous treatment lines and the relative role with respect to other antiangiogenic drugs. As other agents such as immune checkpoint inhibitors are under extensive investigation in oesophago-gastric cancer with promising results (19,20), the antiangiogenic approach is now asked to leave its youth to finally reach mature and reliable efficacy results in this difficult disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Ter Veer E, Haj Mohammad N, van Valkenhoef G, et al. The Efficacy and Safety of First-line Chemotherapy in Advanced Esophagogastric Cancer: A Network Meta-analysis. J Natl Cancer Inst 2016.108. [PubMed]
- Ter Veer E, Haj Mohammad N, van Valkenhoef G, et al. Second- and third-line systemic therapy in patients with advanced esophagogastric cancer: a systematic review of the literature. Cancer Metastasis Rev 2016;35:439-56. [Crossref] [PubMed]
- Fanotto V, Cordio S, Pasquini G, et al. Prognostic factors in 868 advanced gastric cancer patients treated with second-line chemotherapy in the real world. Gastric Cancer 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [Crossref] [PubMed]
- Shen L, Li J, Xu J, et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study). Gastric Cancer 2015;18:168-76. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Yoon HH, Bendell JC, Braiteh FS, et al. Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: a randomized, double-blind, multicenter Phase II trial. Ann Oncol 2016;27:2196-203. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219-25. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]
- Moehler M, Gepfner-Tuma I, Maderer A, et al. Sunitinib added to FOLFIRI versus FOLFIRI in patients with chemorefractory advanced adenocarcinoma of the stomach or lower esophagus: a randomized, placebo-controlled phase II AIO trial with serum biomarker program. BMC Cancer 2016;16:699. [Crossref] [PubMed]
- Yi JH, Lee J, Lee J, et al. Randomised phase II trial of docetaxel and sunitinib in patients with metastatic gastric cancer who were previously treated with fluoropyrimidine and platinum. Br J Cancer 2012;106:1469-74. [Crossref] [PubMed]
- Pavlakis N, Sjoquist KM, Martin AJ, et al. Regorafenib for the Treatment of Advanced Gastric Cancer (INTEGRATE): A Multinational Placebo-Controlled Phase II Trial. J Clin Oncol 2016;34:2728-35. [Crossref] [PubMed]
- A Study of Regorafenib in Refractory Advanced Gastro-Oesophageal Cancer (INTEGRATEII). Accessed on January 4th, 2017. Available online: https://clinicaltrials.gov/show/NCT02773524
- Grothey A, George S, van Cutsem E, et al. Optimizing treatment outcomes with regorafenib: personalized dosing and other strategies to support patient care. Oncologist 2014;19:669-80. [Crossref] [PubMed]
- Hammond WA, Swaika A, Mody K. Pharmacologic resistance in colorectal cancer: a review. Ther Adv Med Oncol 2016;8:57-84. [PubMed]
- Fornaro L, Vasile E, Falcone A. Apatinib in Advanced Gastric Cancer: A Doubtful Step Forward. J Clin Oncol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Moehler M, Delic M, Goepfert K, et al. Immunotherapy in gastrointestinal cancer: Recent results, current studies and future perspectives. Eur J Cancer 2016;59:160-70. [Crossref] [PubMed]
- Kang YK, Satoh T, Ryu MH, et al. Nivolumab (ONO-4538/BMS-936558) as salvage treatment after second or later-line chemotherapy for advanced gastric or gastro-esophageal junction cancer (AGC): A double-blinded, randomized, phase III trial. J Clin Oncol 2017;35:abstr 2.
Cite this article as: Vivaldi C, Falcone A, Fornaro L. Regorafenib: lights and shadows of antiangiogenic therapies in gastric cancer. Transl Gastroenterol Hepatol 2017;2:11.


