Preoperative staging of nodal status in gastric cancer
Introduction
Gastric cancer remains a diagnostic and therapeutic challenge, which is demonstrated to be still one of the highest causes of cancer-related death (1,2). An international consensus agrees that proper staging precedes therapeutic planning and indicates disease prognosis. Besides tumor invasion depth, nodal status remains the most influential parameter indicating survival of gastric cancer (3-5). The probability of lymph node involvement is the major characteristic dividing early from advanced gastric cancer. Lymph node status also dictates the surgical therapy approach. Further, the surgical approach mainly depends on preoperative staging results; and therefore, the accuracy of these results is of the greatest importance (6). The exact prediction of nodal involvement in gastric cancer remains challenging, although different techniques have been evaluated. Hereafter, the most important and latest results regarding preoperative staging of nodal status in gastric cancer are discussed. The basic design of prospective studies regarding this issue evaluates the diagnostic value of certain techniques when comparing preoperative staging results with the pathohistological report of the specimen. Sensitivity is defined as the proportion of detected true-positive nodes among total positive nodal status. Specificity is defined as the proportion of true-negative nodal status among total negative nodes. Accordance of positive and negative nodal status is compared with true-positive and true-negative assessments. Then, a value for sensitivity (also called the true-positive rate) and specificity (also called the true-negative rate) for each diagnostic tool and for the different staging combinations are placed in a 2×2 table. By combining true-positive results with false-positive results, and true-negative with false-negative results, positive and negative predictive values can be illustrated.
Materials and methods
An extensive Medline (through PubMed) search for relevant publications on staging in gastric cancer, restricted to papers published in English, was conducted. Search terms included: gastric OR stomach OR oesophagogastric AND cancer OR carcinoma AND staging OR nodal OR lymph node OR endosonography (or endoscopic ultrasound) OR computed tomography (or CT) OR fluorodeoxyglucose-positron emission tomography (or PET) OR magnetic resonance imaging (or MRI). Due to technical improvements of all imaging methods, the search was mainly focused on studies published within the last 10 years.
Results
Computed tomography (CT)
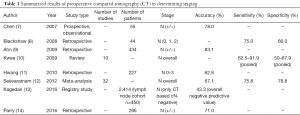
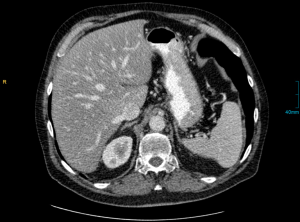
CT is a basic diagnostic tool used for gastric cancer, especially to detect distant metastasis or locally-advanced cancer. Detection of lesions in the lungs and/or liver is crucial for treatment choice and surgical strategies. The accuracy of determining lymph node staging varies between different studies (Table 1). Detection of suspicious lymph nodes by CT mostly depends on the size and localization of the structure (Figure 1). Sensitivity of nodal invasion is reported from 62% to 92% using axial or multi-detector tomography (7-11,15). A current study reviewing 2,414 gastric cancer patients in 116 Canadian institutions categorized 450 patients as “negative nodal status” by CT; these patients underwent resection with curative intent and were actually found to be nodal positive. Thus, the negative predictive value was actually only 43.3% for nodal involvement (13). This means that CT could not detect 56.7% of patients with regional lymph node metastasis. This study provides insights into the lack of accuracy of preoperative CT imaging in determining lymph node staging in nationwide hospitals and emphasizes the importance of staging laparoscopy. Nevertheless, single-institution studies in high-volume centers report a much higher negative predictive value (up to 90.1%) for detecting lymph node staging by preoperative CT in patients with early gastric cancer (9).
Full table
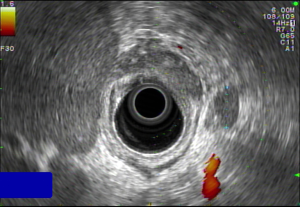
Endoscopic ultrasound (EUS)
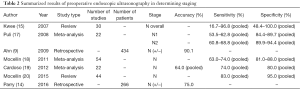
EUS assessment is reported to be the gold standard for determining local lymph node involvement in gastric cancer (16). However, accuracy of this method depends on the investigator’s experience and cannot easily be revised by different investigators. Accuracy is also limited to a certain distance from the transducer. In high-volume centers, the negative predictive value is reported to reach more than 90% (9) (Figure 2). In a prospective database from a high-volume center in the Netherlands, the overall accuracy of detecting nodal status using EUS for both N+ and N− patients is 75% for junctional adenocarcinoma and was proven to be superior compared to CT (14). A meta-analysis which included 22 studies and 1,896 patients reported a pooled sensitivity of 58.2% and 64.9% for patients revealed to be N1 and N2, respectively (17) (Table 2). Unsurprisingly, the more advanced the disease, the higher the accuracy of detection by EUS. Again, these data suggest a non-negligible difference between the results observed by multiple centers and a single high-volume institution.
Full table
Positron emission tomography (PET)
PET, mostly used with 18F-fluoro-2-deoxy-D-glucose (FDG) as a metabolic tracer, has been tested in several studies to reassess if there is an additional staging benefit in gastric cancer (Table 3, Figure 3). In most of these studies, especially earlier ones, no additional benefit, or a questionable benefit which was not proportional to the expenses associated with this method, was found (21,23,26). A crucial point when considering the benefits of FDG-PET assessment is the fact that early investigations only used PET technology, whereas the latest investigations used a combination of FDG-PET and CT (FDG-PET CT), which increases the anatomic accuracy. Furthermore, other tracers are currently being evaluated. A unique characteristic of using PET for staging assessment is that this technique provides both an anatomical and a metabolic image of the cancer. However, PET imaging of gastric cancer is not a standardized staging procedure in many countries because it has a significant lack of overall accuracy. A Japanese study that evaluated the predictive value of FDG-PET CT compared to CT alone for preoperative nodal status in patients with gastric cancer reported an unacceptably low sensitivity of predicting lymph node metastasis (22). The study included 78 patients and reported an overall sensitivity of only 31% for the FDG-PET CT to detect lymph node metastasis preoperatively. The sensitivity for detecting patients with one or two and three lymph node metastasis in the specimen was 31.6% and 12%, respectively, whereas the specificity was reported to be more than 90% overall as well as more than 90% in the subgroups. Interestingly, the sensitivity was reported to be slightly higher when using only CT, indicating that negative glucose enrichment might lead to an underestimation of lymphatic structures. Other characteristics of using FDG-PET in patients with gastric cancer are also concluded in several studies dealing with primary tumors. For both early gastric and diffuse histological type according to the Lauren classification cancers, assessment using FDG-PET-CT does often not yield additional information about preoperative staging. The proportion of FDG-PET-detected positive cancers of diffuse or signet-ring cell subtypes ranges from 0–24%, which also reflects a less reliable assessment of lymph node staging. Studies taking into account the limitations of this technique in gastric cancer can increase the accuracy of FDG-PET-CT. A recent study comparing FDG-PET-CT preoperative staging results to CT alone results of only locally advanced gastric cancers showed an accuracy of 95% for detection of the primary tumor and 86% for predicting lymph node infiltration; results from FDG-PET-CT detection were not significantly superior to CT alone (25). Also, scoring systems that include tumor size, histological subtype, and GLUT-1 immunohistochemical positivity are suggested to improve patient selection for FDG-PET-CT in gastric cancer (27,28).

Full table
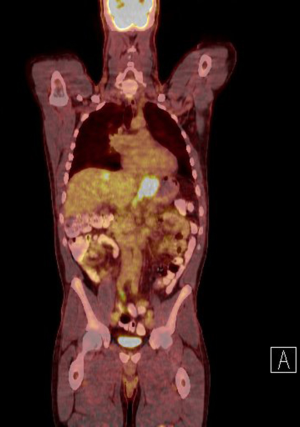
In addition to FDG, 18-fluorothymidine (FLT) has been evaluated as a PET tracer in patients with gastric cancer. In direct comparison to FDG-PET-CT, this tracer delivered the same detection rate of the primary tumor; when compared to staging determined by CT alone, assessment of lymph nodes using FLT was shown to be significantly better regarding sensitivity and specificity (29-31).
Magnetic resonance imaging (MRI)
MRI was found to be helpful regarding the local infiltration of gastric cancer into the stomach wall and possibly the serosa in early studies. However, when compared to other standard techniques like CT or EUS, no benefit or even inferior results were seen regarding the accuracy of lymph node assessment (32,33). One recent comparative study and a recent meta-analysis concluded that MRI was a helpful technique in staging the T-category and had a poor detection rate for lymph node metastasis in gastric cancer (34,35). MRI, when used in a liver-specific protocol, is mostly used to clarify suspicious liver lesions if no biopsy is performed (16).
Discussion
Accurate preoperative lymph node staging in patients with gastric cancer is crucial for planning therapy strategies. For example, staging affects geographical localization of the medical unit, impacts neoadjuvant therapy, determines the extent of resection, affects the extension of lymphadenectomy, and determines the use of minimally invasive techniques. So far, no staging technique is clearly superior and delivers both high sensitivity and specificity when being matched with the pathohistological report. One major reason for this lack of accuracy might be because most staging methods mainly evaluate the size of lymph node structures. A certain lymph node size is then defined as suspicious for lymph node infiltration. Several studies have shown that lymph node size alone is not a reliable factor to predict gastric cancer infiltration. A German study reported that the median size of an infiltrated lymph node is bigger than the size of cancer-free lymph nodes (mean diameter 6.0 vs. 4.1 mm, respectively); however, 55% of infiltrated lymph nodes were smaller than 5 mm (36). Based on these results, it is clear that a staging method that primarily depends on lymph node size will not deliver the desired accuracy, even if all local and distant lymph nodes can be visualized. The most promising technique that delivers the desired qualities for preoperative lymph node staging is PET-CT. Certain tracers, mostly FDG, used for patients with gastric cancer are supposed to visualize tissue metabolism. This assumes that tumor tissue and lymph node metastasis show accelerated or enhanced uptake of the tracer. Due to histological tumor heterogeneity, different levels of metabolic activity in different stages of disease, and different levels of transporter molecules in cancer cells, the use of FDG-PET-CT did not demonstrate a distinct benefit regarding tumor or lymph node staging compared to standard methods such as CT + EUS. More recent studies indicate that appropriate patient selection can lead to a significant improvement of the accuracy of using FDG-PET-CT for lymph node assessment (27,28). For such patient selection, it seems beneficial to include extra features such as GLUT-1 expression; this glucose transporter molecule is important in gastric cancer glucose metabolism (37-39). Other tracers used for PET did not demonstrate reproducible benefits for determining staging so far.
Reflecting on the routinely used preoperative staging methods for nodal status assessment in gastric cancer patients in 2016, it becomes evident that more evaluation is necessary and those new techniques to accurately predict nodal status need to be established. One technique currently being evaluated is near-infrared spectroscopy, which generates dynamic images through the use of an injected fluorescence dye. This method could at least be used for endoscopic or intraoperative assessment of the tumor site and lymph node involvement. Use of near-infrared spectroscopy has shown promising results regarding endoscopic tumor detection (40,41). However, the use of near-infrared spectroscopy to assess lymph nodes in patients with gastric cancer must still be investigated.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Autier P, Boniol M, et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 2007;18:581-92. [Crossref] [PubMed]
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [Crossref] [PubMed]
- Marrelli D, De Stefano A, de Manzoni G, et al. Prediction of recurrence after radical surgery for gastric cancer: a scoring system obtained from a prospective multicenter study. Ann Surg 2005;241:247-55. [Crossref] [PubMed]
- Kim JP, Lee JH, Kim SJ, et al. Clinicopathologic characteristics and prognostic factors in 10 783 patients with gastric cancer. Gastric Cancer 1998;1:125-33. [Crossref] [PubMed]
- Jung H, Lee HH, Song KY, et al. Validation of the seventh edition of the American Joint Committee on Cancer TNM staging system for gastric cancer. Cancer 2011;117:2371-8.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-12. [Crossref] [PubMed]
- Chen CY, Hsu JS, Wu DC, et al. Gastric cancer: preoperative local staging with 3D multi-detector row CT--correlation with surgical and histopathologic results. Radiology 2007;242:472-82. [Crossref] [PubMed]
- Blackshaw G, Lewis WG, Hopper AN, et al. Prospective comparison of endosonography, computed tomography, and histopathological stage of junctional oesophagogastric cancer. Clin Radiol 2008;63:1092-8. [Crossref] [PubMed]
- Ahn HS, Lee HJ, Yoo MW, et al. Diagnostic accuracy of T and N stages with endoscopy, stomach protocol CT, and endoscopic ultrasonography in early gastric cancer. J Surg Oncol 2009;99:20-7. [Crossref] [PubMed]
- Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer 2009;12:6-22. [Crossref] [PubMed]
- Hwang SW, Lee DH, Lee SH, et al. Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J Gastroenterol Hepatol 2010;25:512-8. [Crossref] [PubMed]
- Seevaratnam R, Cardoso R, McGregor C, et al. How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? A meta-analysis. Gastric Cancer 2012;15 Suppl 1:S3-18. [Crossref] [PubMed]
- Kagedan DJ, Frankul F, El-Sedfy A, et al. Negative predictive value of preoperative computed tomography in determining pathologic local invasion, nodal disease, and abdominal metastases in gastric cancer. Curr Oncol. 2016;23:273-9. [Crossref] [PubMed]
- Parry K, Haverkamp L, Bruijnen RC, et al. Staging of adenocarcinoma of the gastroesophageal junction. Eur J Surg Oncol 2016;42:400-6. [Crossref] [PubMed]
- Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: a systematic review. J Clin Oncol 2007;25:2107-16. [Crossref] [PubMed]
- Meyer HJ, Hölscher AH, Lordick F, et al. Current S3 guidelines on surgical treatment of gastric carcinoma. Chirurg 2012;83:31-7. [Crossref] [PubMed]
- Puli SR, Batapati Krishna Reddy J, Bechtold ML, et al. How good is endoscopic ultrasound for TNM staging of gastric cancers? A meta-analysis and systematic review. World J Gastroenterol 2008;14:4011-9. [Crossref] [PubMed]
- Mocellin S, Marchet A, Nitti D. EUS for the staging of gastric cancer: a meta-analysis. Gastrointest Endosc 2011;73:1122-34. [Crossref] [PubMed]
- Cardoso R, Coburn N, Seevaratnam R, et al. A systematic review and meta-analysis of the utility of EUS for preoperative staging for gastric cancer. Gastric Cancer 2012;15 Suppl 1:S19-26. [Crossref] [PubMed]
- Mocellin S, Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev 2015.CD009944. [PubMed]
- Tian J, Chen L, Wei B, et al. The value of vesicant 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) in gastric malignancies. Nucl Med Commun 2004;25:825-31. [Crossref] [PubMed]
- Yun M, Lim JS, Noh SH, et al. Lymph node staging of gastric cancer using (18)F-FDG PET: a comparison study with CT. J Nucl Med 2005;46:1582-8. [PubMed]
- Mukai K, Ishida Y, Okajima K, et al. Usefulness of preoperative FDG-PET for detection of gastric cancer. Gastric Cancer 2006;9:192-6. [Crossref] [PubMed]
- Cui JX, Li T, Xi HQ, et al. Evaluation of (18)F-FDG PET/CT in preoperative staging of gastric cancer: a meta-analysis. Zhonghua Wei Chang Wai Ke Za Zhi 2013;16:418-24. [PubMed]
- Kawanaka Y, Kitajima K, Fukushima K, et al. Added value of pretreatment (18)F-FDG PET/CT for staging of advanced gastric cancer: Comparison with contrast-enhanced MDCT. Eur J Radiol 2016;85:989-95. [Crossref] [PubMed]
- Vallböhmer D, Hölscher AH, Schneider PM, et al. [18F]-fluorodeoxyglucose-positron emission tomography for the assessment of histopathologic response and prognosis after completion of neoadjuvant chemotherapy in gastric cancer. J Surg Oncol 2010;102:135-40. [Crossref] [PubMed]
- Kaneko Y, Murray WK, Link E, et al. Improving patient selection for 18F-FDG PET scanning in the staging of gastric cancer. J Nucl Med 2015;56:523-9. [Crossref] [PubMed]
- Alakus H, Batur M, Schmidt M, et al. Variable 18F-fluorodeoxyglucose uptake in gastric cancer is associated with different levels of GLUT-1 expression. Nucl Med Commun 2010;31:532-8. [PubMed]
- Staniuk T, Małkowski B, Śrutek E, et al. Comparison of FLT-PET/CT and CECT in gastric cancer diagnosis. Abdom Radiol (NY) 2016;41:1349-56. [Crossref] [PubMed]
- Kameyama R, Yamamoto Y, Izuishi K, et al. Detection of gastric cancer using 18F-FLT PET: comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging 2009;36:382-8. [Crossref] [PubMed]
- Herrmann K, Ott K, Buck AK, et al. Imaging gastric cancer with PET and the radiotracers 18F-FLT and 18F-FDG: a comparative analysis. J Nucl Med 2007;48:1945-50. [Crossref] [PubMed]
- Arocena MG, Barturen A, Bujanda L, et al. MRI and endoscopic ultrasonography in the staging of gastric cancer. Rev Esp Enferm Dig 2006;98:582-90. [Crossref] [PubMed]
- Kim AY, Han JK, Seong CK, et al. MRI in staging advanced gastric cancer: is it useful compared with spiral CT? J Comput Assist Tomogr 2000;24:389-94. [Crossref] [PubMed]
- Huang Z, Xie DH, Guo L, et al. The utility of MRI for pre-operative T and N staging of gastric carcinoma: a systematic review and meta-analysis. Br J Radiol 2015;88:20140552. [Crossref] [PubMed]
- Hasbahceci M, Akcakaya A, Memmi N, et al. Diffusion MRI on lymph node staging of gastric adenocarcinoma. Quant Imaging Med Surg 2015;5:392-400. [PubMed]
- Mönig SP, Zirbes TK, Schröder W, et al. Staging of gastric cancer: correlation of lymph node size and metastatic infiltration. AJR Am J Roentgenol 1999;173:365-7. [Crossref] [PubMed]
- Schlößer HA, Drebber U, Urbanski A, et al. Glucose transporters 1, 3, 6, and 10 are expressed in gastric cancer and glucose transporter 3 is associated with UICC stage and survival. Gastric Cancer 2017;20:83-91. [Crossref] [PubMed]
- Berlth F, Mönig S, Pinther B, et al. Both GLUT-1 and GLUT-14 are Independent Prognostic Factors in Gastric Adenocarcinoma. Ann Surg Oncol 2015;22 Suppl 3:S822-31. [Crossref] [PubMed]
- Berlth F, Mönig SP, Schlösser HA, et al. Validation of 2-mm tissue microarray technology in gastric cancer. Agreement of 2-mm TMAs and full sections for Glut-1 and Hif-1 alpha. Anticancer Res 2014;34:3313-20. [PubMed]
- Luo S, Chen C, Mao H, et al. Discrimination of premalignant lesions and cancer tissues from normal gastric tissues using Raman spectroscopy. J Biomed Opt 2013;18:067004. [Crossref] [PubMed]
- Teh SK, Zheng W, Ho KY, et al. Diagnostic potential of near-infrared Raman spectroscopy in the stomach: differentiating dysplasia from normal tissue. Br J Cancer 2008;98:457-65. [Crossref] [PubMed]
Cite this article as: Berlth F, Chon SH, Chevallay M, Jung MK, Mönig SP. Preoperative staging of nodal status in gastric cancer. Transl Gastroenterol Hepatol 2017;2:8.