Lymphadenectomy in elderly/high risk patients: should it be different?
Introduction
Although gastric cancer is showing a decrease in its incidence since the beginning of the 20th century, it still represents the fourth most common type of cancer, and the second leading cause of cancer related death worldwide (1).
Considering its prevalence in the seventh and eighth decade (2) and the global aging of population due to increased life expectancy (3), probably in a near future more elderly patients, with their age-related comorbidities, will be candidates for surgery for gastric carcinoma than in the past (4). This depicts the necessity to define whether guidelines, commonly based on trials involving young and middle-age patients, can be adopted also for the management of this frail population.
Historically, clinical trial enrollment of older adults did not reflect the general population of elderly with cancer because of the low overall numbers of older patients enrolled and the strict inclusion criteria. Hence, study results are often validated only on primarily healthy, “fit” older adults (5), so that it is hard to extrapolate clinical trial data to inform treatment decisions of older patients with cancer (6).
Actually, the prognostic significance of age in gastric cancer is not clearly defined: in literature data are generally limited, and mostly discordant (7,8). Moreover, it is important to underline that overall survival (OS) in the elderly is clearly influenced by coexisting comorbidities. When disease-specific survival (DSS) is analyzed, it seems that prognosis in the elderly patients is not worse than in the control group (9-11), and even when it results to be worse, it is often a consequence of a more advanced disease at the time of diagnosis (8).
In this review, we aim to expose evidences in the role of lymphadenectomy for gastric cancer in elderly patients; in facts, if the gold standard for curative gastric surgery requires an extended (D2) lymphadenectomy, a wide number of limited lymphadenectomy is still performed, especially in elderly patients. Actually, guidelines (12,13) do not specify any limit deriving from age and/or comorbidities, and the choice is usually based on the surgeons’ selection.
In order to clarify the impact of extended lymphadenectomy on both short-term and long-term outcomes of elderly/high risk patients, we reviewed literature concerning this issue.
Methods
We evaluated recent literature (from 2005 up to June 2016) on PubMed Central with combination of following MESH terms: gastric cancer and elderly, elderly and gastric cancer and lymphadenectomy, elderly and gastric cancer and complications, high risk patients and gastric cancer. All abstracts were read separately by two different surgeons, and scientific relevance of papers has been assessed according to originality of the article, accuracy of statistical method, number of patients. All of the selected papers were fully read by three or more surgeons, and only papers reported in references have been judged clinically and scientifically relevant. Table 1 reports data from some of the considered studies with specific focus on morbidity and mortality of elderly patients.
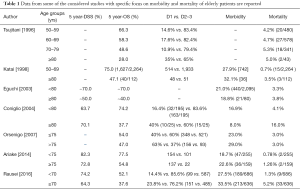
Full table
Who are elderly patients?
Although in last years literature reports many studies concerning oncologic gastric surgery in elderly patients, there is still no homogeneity in the definition of this subgroup, and an established cut-off age seems far to be determined: in facts, if some authors defined as elderly patients with more than 65 years (14), other authors reported thresholds of 70, 75 or 80 years (3,11,15-17), so that it is not easy to compare results from different studies.
Moreover, considering improvement in quality of life in developed countries with a subsequent increase of mean life expectancy, and with a discrete part of old population being affected by one or more comorbidities, it seems that a criterion purely based on age would not fit the aim of being a discriminating parameter (18).
Lim reported a higher rate of comorbidities in the elderly, with a significant increase in the number of comorbidities with aging (8), underlining the frailty of this population. Nonetheless, aging is an individualized process, bringing both physiologic and psychosocial changes that can affect tumor biology, decision-making and tolerance for cancer treatment, and ultimately outcomes (18).
The prevalence of multiple chronic illnesses in the Unites States is increasing in older adults (19) and it has been observed an even higher prevalence of comorbidity in elderly cancer patients than in an age-matched control group (20,21). In this context, different scoring systems for geriatric assessment have been developed and several reliable indexes are available not only to measure comorbidity in cancer patients, but also with the aim to describe different aspects of geriatric oncologic patients, from nutrition to psychological status to cognition (22,23). All of these indexes show that globally comorbidity is a strong predictor of outcome (24). Surgeons should introduce the use of such indexes as a routine in their clinical practice, in order to fill the gap of evidence in geriatric oncology in next future, and finally clarify the best approach in elderly patients (25).
Characteristics of gastric cancer in the elderly
Several distinguishing features have been reported to differ between gastric cancer in the elderly and in the younger patients, although results are sometimes discordant.
At first, gastric cancer in the elderly presents a higher predominance in males, in contrast to the younger group (7,11,26). Concerning macroscopical features, many studies reported a prevalence of tumor in the lower third of the stomach among the elderly (9,27). Also gross appearance seems to differ from that of young patients, with a higher prevalence of superficial depressed type in the elderly when early gastric cancer is considered, and a prevalence of ulcerative tumors (according to Borrmann’s classification), in contrast to the prevalence of the diffuse infiltrative type in the young population (26). Moreover, an increase of the differentiated type together with aging has been described (11), and considering early stages of disease, poorly differentiated and signet ring-cell carcinomas account for only 10% of the cases (26).
Again, older patients present a higher rate of synchronous gastric carcinoma (up to 15% of cases); many authors suggested that the higher incidence of intestinal-type according to Lauren and the underlying atrophic gastritis could explain the higher rate of multifocal cancerogenesis (7).
Regarding lymph node involvement, if several reports did not show any significant difference in the rate of lymph node involvement (10,28-30), other authors described a trend toward higher incidence of lymph node involvement in the elderly, although statistical significance was not reached (8,31); conversely in cases of early disease, several studies report lower prevalence of lymph node metastases in the elderly as compared to patients aged under 75 (7,27).
The pattern of distant metastases also shows some differences in comparison to the younger group: probably because of a higher prevalence of well differentiated carcinoma—associated with a higher prevalence of vascular invasion, hematogenous metastases are more common in the elderly, while peritoneal involvement has a lower frequency (7).
Extent of lymphadenectomy in elderly gastric cancer patients
Different RCTs in latest years compared the benefit on survival of extended (D2) versus limited (D1) lymphadenectomy in gastric cancer patients, and gastrectomy with D2 lymphadenectomy is now codified as gold standard in the treatment of non-early (T ≥2 every N M0, T1N + M0) tumor in both Eastern and Western world, without any limitation regarding age and/or comorbidities (12,13). D2 lymphadenectomy, however, has a high operation-related death rate and is thought to be risky in elderly patients (32,33). Therefore many surgeons are reluctant to have elderly patients undergone extended gastric surgery (3,34,35), although a meta-analysis showed that perioperative mortality, still high in patients aged >80 years, is progressively decreasing in recent years (2).
The role of age as a prognostic factor in patients who underwent curative surgery for gastric cancer is still debated. When considering OS, elderly patients report low survival rate if compared to young patients, reflecting their frailty and the effect of their comorbidities probably more than the neoplastic disease itself (36). DSS instead, does not seem to be worse than that in younger patients, even if less aggressive surgery is more frequently performed in elderly patients, as a selection bias of the surgeons (34,35). Moreover, when a worse prognosis has been observed in the elderly, it seemed that it was due to a more advanced stage of disease at the time of diagnosis (36), rather than to a more aggressive biology of the tumor. This further complicates the analysis of the relationship between survival and complications.
The revision of the Dutch trial investigating the causes of high postoperative morbidity with worsening OS observed after D2 lymphadenectomy, reports age to be consistently associated with negative short-term outcome (37).
Nienhueser and colleagues instead found that, although postoperative medical complications were more frequent in patients aged more than 70 years, multivariate analysis did not select age as a prognostic factor for surgical complications, which were instead related to ASA III–IV, abdomino-thoracic resection and pre-existing comorbidities, indicating that age alone is no contraindication for resection of gastric cancer if the patient fits for extended surgery (38).
Also Kodera, when comparing results of D2–D3 lymphadenectomy for gastric cancer in patients aged more or less than 65 years (but excluding patients aged more than 75 years) did not find age to influence overall complications rate, but he observed that elderly patients experienced a significantly higher rate of severe complications (14).
If preoperative risk factors were found to be more common among elderly patients, postoperative complications did not seem to occur with a significant higher frequency in this subgroup of patients, or at least this is true for surgical complications (16,34-36); however, it seems that surgery was less aggressive in these studies, with a low number of elderly patients underwent to extended lymphadenectomy. However, in patients aged more than 75 years, Eguchi observed that, when extended lymphadenectomy was performed both morbidity and mortality resulted to be higher if compared to elderly patients treated with limited lymph node dissection (39).
In a study by Coniglio comparing surgical treatment in patients aged more or less than 80 years, the higher ASA risk reported among the elderly did not influence the rate of curative resection, although a higher number of limited lymphadenectomy was observed in this group. Cumulative morbidity and mortality did not significantly differ between young and elderly patients, and both univariate and multivariate analysis selecting ASA risk (and not age) as a prognostic factor for survival, thus proposing to modulate the extent of lymphadenectomy on the basis of cancer stage, considering an aprioristic aggressive attitude just for younger patients (3).
Concerning survival, even if the Taiwanese trial, comparing limited versus extended lymphadenectomy, can be only partially considered for the aim of our review (as they excluded patients aged >75 years), it should be noticed that age >65 years resulted not to be a prognostic factor in both uni- and multivariable analysis of survival (40). The same results have been reported in the British trial, which included patients up to 85 years, with no significant difference in survival of young (<65) versus elderly patients (≥65 years) (41).
Many studies showed similar DSS rates when comparing the efficacy of D2 versus D1 in both young and elderly gastric cancer patients (3,11,16), although paradoxically a recent Italian RCT described a better DSS in patients aged ≥70 years after D1 lymphadenectomy (42).
When considering specifically OS, which reflects real survival of patients and is potentially more influenced by postoperative complications, Literature presents controversial results. If Degiuli found no significant difference in OS after D1 versus D2 lymph node resection in elderly patients (42), Nienhueser, observed a worsening in OS only for patients aged more than 80 years, with no statistically significant differences in surgical complications between patients aged more or less than 80 years (38).
Liang, comparing prognosis of gastric cancer patients aged more than 70 years, observed no significant differences in both OS and DSS between young and old patients; he also reported a lower number of extended lymphadenectomy in the elderly group, concluding that due to short life expectancy in people aged more than 70 years, a limited lymph node dissection (D1) is appropriate in this patients, and stated postoperative chemotherapy is possibly unnecessary for the elderly (11).
Attempting to investigate the relationship among age/comorbidities, complications and survival, scoring systems resulted to be useful. The Estimation of Physiological Ability and Surgical Stress (E-PASS) scoring system (43), has been used to further investigate elderly patients underwent surgery for gastric cancer. Ariake et al. reported a preoperative risk score to be significantly higher in patients aged more than 75 years, while surgical stress was similar between the two groups. Preoperative score together with surgical stress showed to be significant prognostic factor for postoperative death both at univariate and multivariate analysis, leading the author to suggest less invasive surgery in elderly high risk patients (17).
Similarly, in our recent multicentre retrospective study on behalf of the Italian Research Group for Gastric Cancer (IRGGC), we applied a further stratification using Charlson Comorbidity Score (CCS) to individuate subgroups of elderly patients who may actually benefit from an extended lymphadenectomy, without increasing postoperative morbidity and mortality. We evaluated the safety of curative gastrectomy with extended lymphadenectomy in elderly and high risk patients, confirming the efficacy of D2 lymph node dissection in determining better survival rates in gastric cancer patients. However, after extended nodal dissection in highly co-morbid elderly patients, even when nodal involvement was present, OS did not show clear benefits owing to the high risk of perioperative complications, leading to conclude that in selected geriatric patients limited lymphadenectomy (with the removal of more than 15 nodes) is still an option not to be refused a priori (9).
Possible confounding factors
Although literature showed increasing interest in surgical treatment of gastric cancer in the elderly in recent years, there is still no consensus on the management of this subgroup of patients. Results from different studies are difficult to compare, as the cut off for the definition of a patient as “old” is not homogeneous, and comorbidities are calculated through several different scoring system.
Moreover, many authors report a lower rate of extended lymphadenectomy in the elderly group, with up to 50% of these patients underwent adequate lymph node dissection as recommended in Japanese guidelines (17). Finally, the role of perioperative chemotherapy cannot be easily evaluated, both because of the administration of different regimens with different schedules and because of the frequent exclusion of elderly high risk patients from oncological trials (18).
Conclusions
The extent of lymphadenectomy in elderly/high risk patients is still a matter of debate. An accurate preoperative evaluation of patients and an optimization of general conditions, together with an accurate staging of disease, are obviously necessary to tailor on patients the best surgical option, in order to avoid the risk of under-treatment in elderly patients who might benefit from a more aggressive approach.
If extended lymphadenectomy seems to be a safe and effective procedure in fit elderly patients, results from different reports suggest a limited lymph node dissection in selected geriatric gastric cancer patients (9,11,39), in order to reduce postoperative complications’ rate that probably influences OS in elderly.
However, further studies are needed to gain evidence in the delicate field of geriatric surgical oncology.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferro A, Peleteiro B, Malvezzi M, et al. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer 2014;50:1330-44. [Crossref] [PubMed]
- Kranenbarg EK, van de Velde CJ. Gastric cancer in the elderly. Eur J Surg Oncol 1998;24:384-90. [Crossref] [PubMed]
- Coniglio A, Tiberio GA, Busti M, et al. Surgical treatment for gastric carcinoma in the elderly. J Surg Oncol 2004;88:201-5. [Crossref] [PubMed]
- Ramesh HS, Pope D, Gennari R, et al. Optimising surgical management of elderly cancer patients. World J Surg Oncol 2005;3:17. [Crossref] [PubMed]
- Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341:2061-7. [Crossref] [PubMed]
- Crome P, Flanagan RJ. Pharmacokinetic studies in elderly people. Are they necessary? Clin Pharmacokinet 1994;26:243-7. [Crossref] [PubMed]
- Saif MW, Makrilia N, Zalonis A, et al. Gastric cancer in the elderly: an overview. Eur J Surg Oncol 2010;36:709-17. [Crossref] [PubMed]
- Lim JH, Lee DH, Shin CM. J Korean Med Sci 2014;29:1639-45. [Crossref] [PubMed]
- Rausei S, Ruspi L, Rosa F, et al. Extended lymphadenectomy in elderly and/or highly co-morbid gastric cancer patients: A retrospective multicenter study. Eur J Surg Oncol 2016;42:1881-1889. [Crossref] [PubMed]
- Kitamura K, Yamaguchi T, Taniguchi H, et al. Clinicopathological characteristics of gastric cancer in the elderly. Br J Cancer 1996;73:798-802. [Crossref] [PubMed]
- Liang YX, Deng JY, Guo HH, et al. Characteristics and prognosis of gastric cancer in patients aged ≥ 70 years. World J Gastroenterol 2013;19:6568-78. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23. [Crossref] [PubMed]
- De Manzoni G, Marrelli D, Baiocchi GL, et al. The Italian Research Group for Gastric Cancer (GIRCG) guidelines for gastric cancer staging and treatment: 2015. Gastric Cancer 2017;20:20-30. [PubMed]
- Kodera Y, Sasako M, Yamamoto S, et al. Identification of risk factors for the development of complications following extended and superextended lymphadenectomies for gastric cancer. Br J Surg 2005;92:1103-9. [Crossref] [PubMed]
- Degiuli M, Sasako M, Ponti A, et al. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg 2010;97:643-9. [Crossref] [PubMed]
- Kunisaki C, Akiyama H, Nomura M, et al. Comparison of surgical outcomes of gastric cancer in elderly and middle-aged patients. Am J Surg 2006;191:216-24. [Crossref] [PubMed]
- Ariake K, Ueno T, Takahashi M, et al. E-PASS comprehensive risk score is a good predictor of postsurgical mortality from comorbid disease in elderly gastric cancer patients. J Surg Oncol 2014;109:586-92. [Crossref] [PubMed]
- Mohile S, Dale W, Hurria A. Geriatric oncology research to improve clinical care. Nat Rev Clin Oncol 2012;9:571-8. [Crossref] [PubMed]
- Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med 2002;162:2269-76. [Crossref] [PubMed]
- Jørgensen TL, Hallas J, Friis S, et al. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br J Cancer 2012;106:1353-60. [Crossref] [PubMed]
- Koroukian SM, Murray P, Madigan E. Comorbidity, disability, and geriatric syndromes in elderly cancer patients receiving home health care. J Clin Oncol 2006;24:2304-10. [Crossref] [PubMed]
- Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA Cancer J Clin 2010;60:120-32. [Crossref] [PubMed]
- Elsawy B, Higgins KE. The geriatric assessment. Am Fam Physician 2011;83:48-56. [PubMed]
- Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer 2000;36:453-71. [Crossref] [PubMed]
- Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol 2016;7:249-57. [Crossref] [PubMed]
- de Manzoni G, Roviello F, Siquini W. editors. Surgery in the multimodal management of gastric cancer. Milan, Italy: Springer-Verlag Italia, 2012.
- Arai T, Esaki Y, Inoshita N, et al. Pathologic characteristics of gastric cancer in the elderly: a retrospective study of 994 surgical patients. Gastric Cancer 2004;7:154-9. [Crossref] [PubMed]
- Saito H, Osaki T, Murakami D, et al. Effect of age on prognosis in patients with gastric cancer. ANZ J Surg 2006;76:458-61. [Crossref] [PubMed]
- Kim DY, Joo JK, Ryu SY, et al. Clinicopathologic characteristics of gastric carcinoma in elderly patients: a comparison with young patients. World J Gastroenterol 2005;11:22-6. [Crossref] [PubMed]
- Maehara Y, Emi Y, Tomisaki S, et al. Age-related characteristics of gastric carcinoma in young and elderly patients. Cancer 1996;77:1774-80. [Crossref] [PubMed]
- Wang JY, Hsieh JS, Huang CJ, et al. Clinicopathologic study of advanced gastric cancer without serosal invasion in young and old patients. J Surg Oncol 1996;63:36-40. [Crossref] [PubMed]
- Katai H, Sasako M, Sano T, et al. Gastric cancer surgery in the elderly without operative mortality. Surg Oncol 2004;13:235-8. [Crossref] [PubMed]
- Katai H, Sasako M, Sano T, et al. The outcome of surgical treatment for gastric carcinoma in the elderly. Jpn J Clin Oncol 1998;28:112-5. [Crossref] [PubMed]
- Orsenigo E, Tomajer V, Palo SD, et al. Impact of age on postoperative outcomes in 1118 gastric cancer patients undergoing surgical treatment. Gastric Cancer 2007;10:39-44. [Crossref] [PubMed]
- Tsujitani S, Katano K, Oka A, et al. Limited operation for gastric cancer in the elderly. Br J Surg 1996;83:836-9. [Crossref] [PubMed]
- Eguchi T, Fujii M, Takayama T. Mortality for gastric cancer in elderly patients. J Surg Oncol 2003;84:132-6. [Crossref] [PubMed]
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [Crossref] [PubMed]
- Nienhueser H, Kunzmann R, Sisic L, et al. Surgery of gastric cancer and esophageal cancer: Does age matter? J Surg Oncol 2015;112:387-95. [Crossref] [PubMed]
- Eguchi T, Takahashi Y, Ikarashi M, et al. Is extended lymph node dissection necessary for gastric cancer in elderly patients? Eur J Surg 2000;166:949-53. [Crossref] [PubMed]
- Wu CW, Hsiung CA, Lo SS, et al. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol 2006;7:309-15. [Crossref] [PubMed]
- Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 1999;79:1522-30. [Crossref] [PubMed]
- Degiuli M, Sasako M, Ponti A, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg 2014;101:23-31. [Crossref] [PubMed]
- Haga Y, Ikei S, Ogawa M. Estimation of Physiologic Ability and Surgical Stress (E-PASS) as a new prediction scoring system for postoperative morbidity and mortality following elective gastrointestinal surgery. Surg Today 1999;29:219-25. [Crossref] [PubMed]
Cite this article as: Ruspi L, Galli F, Pappalardo V, Inversini D, Martignoni F, Boni L, Dionigi G, Rausei S. Lymphadenectomy in elderly/high risk patients: should it be different? Transl Gastroenterol Hepatol 2017;2:5.


