Classification of nodal stations in gastric cancer
Introduction
The Japanese Research Society for Gastric Cancer (JRSGC) published the first edition of the “General Rules for Gastric Cancer Study” in 1973 (1); as a matter of fact, several lymph node (LN) studies performed in this country in the middle of last century revealed pathways of LN drainage.
According to these studies, in the first English edition of “the General Rules of JRSGC” (2), which were widely accepted and adopted in many Western countries, regional LNs for gastric cancer (GC) were classified into 16 stations according to their location (Table 1, Figure 1).
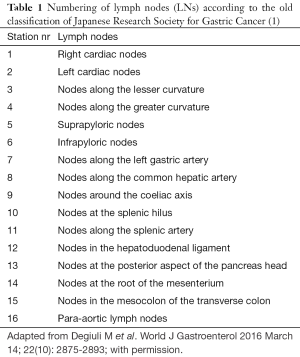
Full table
In 1997, the JRSGC was transformed into the Japanese Gastric Cancer Association (JGCA) and this new association has maintained its commitment to the concept of the Japanese classification.
In the newest classification of the JGCA, there is a very comprehensive description of regional LNs (3).
Although a decrease in incidence in the last years, GC still represents one of the most common causes of cancer death in the world (4). In Western countries, early diagnosis is very uncommon and the incidence of nodal metastases is high (4). Even in early GC, the incidence of LN metastasis exceeds 10%; it was reported to be 14.1% overall and was 4.8% to 23.6% depending on cancer depth (5). Preoperative work up is crucial to evaluate carefully LN status and to provide the patient the best chance of cure; however, sufficient results are not being obtained using various modalities. Surgery remains the mainstay treatment for cure or long-term survival for GC. Surgical resection and LN dissection give a chance of cure in case of local disease without distant metastasis. However, there is no survival benefit from surgery for systemic disease with distant metastasis, such as para-aortic LN metastasis, except for palliation (6). Therefore, the extent of disease represents one of the most important prognostic indicators for GC, and the importance of extended lymphadenectomy is still a matter of debate. The concept of micro-metastasis has been described as a prognostic factor (7-12), and the biological mechanisms of LN metastasis are currently under investigation (13-15).
The strategy of LN dissection is based on a perfect knowledge of the vascular anatomy of the upper abdominal cavity, because arteries and veins represent essential landmarks in the operating fields.
We will describe systematically all the locoregional LN stations in GC, with particular regard to their anatomical and vascular boundaries (16).
Classification
Anatomical definition of LNs and LN locations
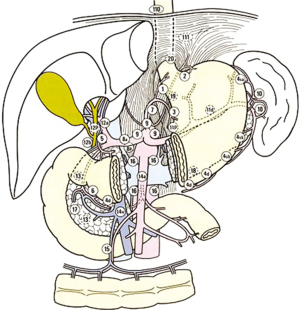
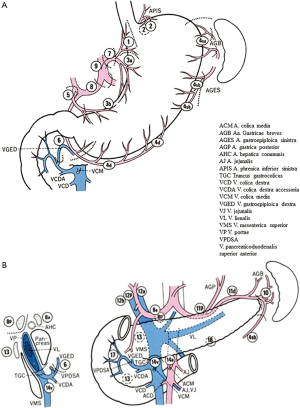
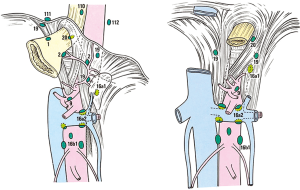
The regional LNs of the stomach are classified into stations numbered from 1 to 20 (Table 2), plus stations 110, 111 and 112. Some of LN stations from 1 to 20 have been distinguished in other subgroups of nodes (Figures 2A,B,3). LN stations 1–12 and LN station 14v are defined as regional stations; the other LN locations are considered as distant stations and in case of metastases are classified as M1. LNs No. 16, 19, 10, 110 and 111 are considered as regional LN in case of direct invasion of the esophagus by the tumor (Figure 4).

Full table
At moment, LN metastasis (N) are classified as follows: (I) NX: when regional LNs cannot be assessed; (II) N0: when there are no regional LNs metastasis; (III) N1: in case of 1–2 regional LNs metastasis; (IV) N2: in case of 3–6 regional LNs metastasis; and (V) N3: in case of 7 or more regional LNs metastasis (N3a: metastasis in 7–15 regional LNs; N3b: metastasis in >15 regional LNs).
In 2011 the JGCA published the GC treatment guidelines based on the 3rd English edition of the Japanese Classification of GC (3), which defined the extent of systematic LN dissection according to the type (distal or total) of gastric resection indicated.
Anatomical borders of locoregional LNs
Perigastric LNs
Station number 1 (right paracardial nodes)
Right paracardial LNs are located on the right side of the cardia, along the first ramification of the ascending branch of the left gastric artery (cardio-esophageal branch).
Station number 2 (left paracardial nodes)
Left paracardial LNs are located on the left side of the cardias, along the cardio-oesophageal branch of the left inferior phrenic vessels.
Station number 3 (lesser curvature nodes)
Lesser curvature LNs are located along the descending branch of the left gastric artery and along the right gastric artery distal to the first gastric branch.
Station number 4 (greater curvature nodes)
Greater curvature LNs are divided into two groups separated by the Von Ghoete point, where right and left gastroepiploic arteries meet each other at full channel: a left group (4s) and a right group (4d) .
Moreover, the left group is divided into a proximal part (4sa) and a distal part (4sb). LNs of the proximal part of left group 4 (4sa) are located around the short gastric vessels while LNs of the distal part (4sb) are located along the left gastroepiploic vessels. LNs of the right part of group 4 are located along the right gastroepiploic vessels, distal to the first gastric branch (boundary with infrapyloric LNs).
Station number 5 (suprapyloric nodes)
Suprapyloric LNs are located at the lesser curvature, immediately above the pylorus, along the right gastric artery and its origin.
Station number 6 (infrapyloric nodes)
Infrapyloric LNs are located at the greater curvature, immediately below the pylorus, at the confluence of the right gastroepiploic vein with the anterior inferior pancreaticoduodenal vein.
Second tier LNs
Station number 7 (left gastric artery nodes)
Left gastric artery LNs are located along the left gastric artery (Figure 3), between its roots from the coeliac trunk till the origin of its ascending branch on the lesser curvature.
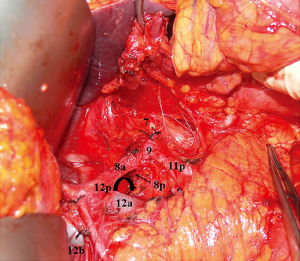
Station number 8 (common hepatic artery nodes)
Common hepatic artery LNs are located around the common hepatic artery from its root from the coeliac trunk to the branching off of the gastroduodenal artery. These LNs are distinguished into an anterior part (8a), and a posterior part (8p) (Figure 3).
Station number 9 (coeliac trunk nodes)
Coeliac trunk LNs are located immediately near the origins of the left gastric artery, the common hepatic artery, and the splenic artery (Figure 3).
Station number 11 (splenic artery nodes)
Splenic artery LNs are located along the splenic artery and are distinguished into a proximal group (11p) and a distal group (11d):
- 11p: from the origin of the splenic artery from the celiac axis, to the rise of the posterior gastric artery, about halfway between its origin and the pancreatic tail end;
- 11d: around the splenic artery from the origin of the posterior gastric artery to the end of the pancreatic tail (Figure 3).
Second tier or third tier or M nodes (according to the site of the primary tumor)
Station number 10 (splenic hilum nodes)
Splenic hilum LNs are located at the splenic hilum, distal to the end of the pancreatic tail. The vascular boundary between 10 and 4sb LNs is represented by the first gastric ramification of the left gastroepiploic.
Station number 12 (hepatoduodenal ligament nodes)
Hepatoduodenal ligament LNs are located in the context of this ligament and include three separate groups:
- 12a: left hepatoduodenal ligament LNs, located along the proper hepatic artery, in the caudal half between the confluence of the right and left hepatic ducts and the upper pancreatic margin;
- 12b and 12p: posterior hepatoduodenal ligament LNs, again divided into nodes located along the bile duct in the caudal half between the confluence of the right and left hepatic ducts and the upper pancreatic margin (12b) and into nodes located posteriorly to the portal vein in the caudal half between the confluence of the right and left hepatic ducts and the upper pancreatic margin (12p) (Figure 3).
Station number 13 (retropancreatic nodes)
Retropancreatic LNs are located on the posterior surface of the head of the pancreas cranial to the Vater’s ampulla along the superior and inferior branches of the posterior pancreaticoduodenal artery. The left lateral border of this location is marked by the portal vein, while the upper border is represented by the origin of posterior hepatoduodenal ligament LNs.
Station number 14 [superior mesenteric vein (VMS) and artery (AMS) nodes]
VMS and AMS LNs are located along the confluence of the VMS (14v) into the portal vein and along the origin of the AMS (14a), at the root of the mesenterium. The lateral border is represented by the confluence of the gastrocolic vein (TGC) into the VMS; the lower border is located at the confluence of the middle colic vein into the VMS and the upper border is represented by the origin of the AMS from the aorta, at the lower hedge of the pancreas.
Station number 15 (middle colic nodes)
Middle colic LNs are located in the transverse mesocolon around the middle colic artery and vein, from their origin/confluence from/into the superior mesenteric vessels, till the mesocolic hedge of the transverse colon.
Station number 16 (aortic hiatus—a1, middle—a2/b1 and caudal—b2 para-aortic nodes)
Station number 16 includes four separate groups of LNs around the abdominal aorta and inferior vena cava:
- 16a1: around the diaphragmatic aortic hiatus, over the anterior side of the aorta, from the inferior hedge of the hiatus to the upper border of the coeliac artery;
- 16a2: over the anterior surface of the aorta, from the coeliac artery to the lower border of the left renal vein;
- 16b1: around the anterior surface of the aorta and vena cava, from the lower border of the left renal vein to the upper border of the origin of the inferior mesenteric artery; right and left border are defined by the right border of the inferior vena cava and by the left gonadic vessels;
- 16b2: around the anterior surface of the aorta and vena cava, between the upper border of the origin of the inferior mesenteric artery and the aortic bifurcation (Figure 4).
Station number 17 and 18 (peripancreatic nodes)
Peripancreatic LNs are located along pancreaticoduodenalis superior anterior vessels, on the anterior surface of the pancreatic head beneath the pancreatic sheath (station 17) and along the inferior border of the pancreatic body (station 18) (Figure 2B).
Station number 19 (infradiaphragmatic)
Infradiaphragmatic LNs are located in the infradiaphragmatic space predominantly along the subphrenic vessels (Figure 4).
Station number 20 (paraesophageal)
Paraesophageal LNs are located in the paraesophageal region in the diaphragmatic esophageal hiatus (Figure 4).
Station number 110, 111 and 112 (lower thorax)
These LNs are located in the lower thorax [110], in the supradiaphragmatic space separate from the esophagus [111], and in the posterior mediastinal space separate from the esophagus and the esophageal hiatus [112], respectively (Figure 4).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Japanese Research Society for Gastric Cancer. The general rules for The gastric cancer study in surgery. Jpn J Surg 1973;3:61-71. [Crossref] [PubMed]
- Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn J Surg 1981;11:127-39. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-12. [Crossref] [PubMed]
- Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol 2006;20:633-49. [Crossref] [PubMed]
- Roviello F, Rossi S, Marrelli D, et al. Number of lymph node metastases and its prognostic significance in early gastric cancer: a multicenter Italian study. J Surg Oncol 2006;94:275-80; discussion 274. [Crossref] [PubMed]
- Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 2008;359:453-62. [Crossref] [PubMed]
- Yasuda K, Adachi Y, Shiraishi N, et al. Prognostic effect of LN micrometastasis in patients with histologically node-negative gastric cancer. Ann Surg Oncol 2002;9:771-4. [Crossref] [PubMed]
- Siewert JR, Kestlmeier R, Busch R, et al. Benefits of D2 lymph node dissection for patients with gastric cancer and pN0 and pN1 lymph node metastases. Br J Surg 1996;83:1144-7. [Crossref] [PubMed]
- Fukagawa T, Sasako M, Mann GB, et al. Immunohistochemically detected micrometastases of the lymph nodes in patients with gastric carcinoma. Cancer 2001;92:753-60. [Crossref] [PubMed]
- Kikuchi Y, Tsuchiya A, Ando Y, et al. Immunohistochemical detection of lymph node microinvolvement in node-negative gastric cancer. Gastric Cancer 1999;2:173-178. [Crossref] [PubMed]
- Nakajo A, Natsugoe S, Ishigami S, et al. Detection and prediction of micrometastasis in the lymph nodes of patients with pN0 gastric cancer. Ann Surg Oncol 2001;8:158-62. [Crossref] [PubMed]
- Cai J, Ikeguchi M, Maeta M, et al. Clinicopathological value of immunohistochemical detection of occult involvement in pT3N0 gastric cancer. Gastric Cancer 1999;2:95-100. [Crossref] [PubMed]
- Kitadai Y, Kodama M, Cho S, et al. Quantitative analysis of lymphangiogenic markers for predicting metastasis of human gastric carcinoma to lymph nodes. Int J Cancer 2005;115:388-92. [Crossref] [PubMed]
- Yonemura Y, Endo Y, Fujita H, et al. Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cancer Res 1999;5:1823-9. [PubMed]
- Amioka T, Kitadai Y, Tanaka S, et al. Vascular endothelial growth factor-C expression predicts lymph node metastasis of human gastric carcinomas invading the submucosa. Eur J Cancer 2002;38:1413-9. [Crossref] [PubMed]
- Degiuli M, De Manzoni G, Di Leo A, et al. Gastric cancer: Current status of lymph node dissection. World J Gastroenterol 2016;22:2875-93. [Crossref] [PubMed]
Cite this article as: Rosa F, Costamagna G, Doglietto GB, Alfieri S. Classification of nodal stations in gastric cancer. Transl Gastroenterol Hepatol 2017;2:2.


