Gastric neuroendocrine tumor (NET): report of one case
Case presentation
Disease history
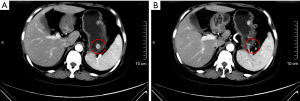
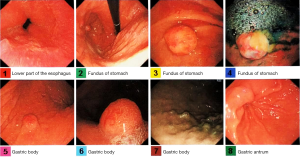
The patient is a 41-year-old woman. On November 27, 2013, the patient visited our center, in which abdominal contrast-enhanced CT (Figure 1) revealed a rounded hypertense lesion sized 1.3 cm × 0.9 cm inside the stomach. On December 5, 2013, gastroscopy in other hospital (Figure 2) showed multiple gastric eminent lesions; a polypoid uplift sized about 1.5 cm was seen in the fundus of stomach; multiple polypoid uplifts sized 0.2–1.5 cm were found in the greater gastric curvature. Pathology in our hospital showed NETs (G1) in the fundus of stomach (Ki-67, +2%; mitotic figure, <2/10 HPF). In the upper portion of the stomach, there was chronic inflammation of mucosa; scattered multifocal spherical proliferation of neuroendocrine cells was seen in the proper layer, which was discrete and not fused. Proliferation of neuroendocrine cells was considered. Immunohistochemistry showed: in the gastric fundus, CgA (+), Syn (+), Ki-67 (+2%). The diagnosis was gastric NETs. She sought treatment in the Department of Integrated Chinese and Western medicine and Oncology in our center on December 16, 2013.
Laboratory tests at admission showed: serum gastrin, 514.01 pg/mL (normal range, <100 pg/mL), serum CgA, 2,051.4 ng/mL (normal range, <100 ng/mL); anti-gastric parietal cell antibody, positive; serum vitamin B12, 120 pmol/L (normal range, 133–675 pmol/L); and CEA and NSE levels, normal. The thyroid function was normal, and both TPO-Ab and TG-Ab were negative. The general condition of the patient was good, although there was occasionally belching after a meal. Urination and defecation were normal.
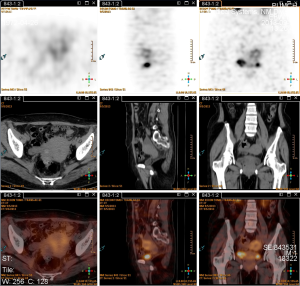
On September 5, 2013, somatostatin receptor scintigraphy in other hospital (Figure 3) showed gastric lesions without radioactive uptake; there was no expression of somatostatin receptor, and no abnormal radioactive uptake was found throughout the body.
On December 18, 2013, endoscopic ultrasonography (Figure 4) showed a 1.5-cm polypoid uplift in the fundus of stomach, along with multiple flat or polypoid uplifts sized 0.2–1.0 cm in the gastric body. The lesion (sized 1.3 cm × 1.0 cm) in the fundus of stomach invaded the mucosa and submucosa, with uneven echoes; its borders were relatively clear.
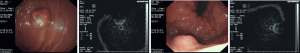
The proper muscular layer was relatively intact and continuous. The multiple submucosal uplifts in the gastric body arise from the gap between mucosa and submucosa, with even and slightly strong echoes. The proper muscular layer was continuous.
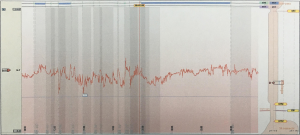
On December 19, 2013, the 24-hour intragastric pH monitoring (Figure 5) showed that the percentage of time with pH below 4.0 was 0, suggesting achlorhydria.
Previous disease history: in August 2013, she underwent laparoscopic radical operation for rectal cancer in other hospital. Pathology showed a rectal NET (G1) sized 2.0 cm × 1.7 cm × 1.2 cm invaded the muscular layer, with metastases in peri-rectal lymph nodes (3/5). Immunohistochemistry showed: CgA (−), Syn (+), and Ki-67 (+2%).
On November 27, 2013, she received routine abdominal and pelvic contrast-enhanced CT in our hospital, during which no metastasis was found; however, intragastric lesions were detected.
On December 23, 2013, MDT consultation on NETs in our center was performed. In the patient, gastroscopy showed the presence of multiple polypoid lesions (sized 0.2–1.5 cm) in the fundus and body of the stomach; serum gastrin elevated; autoimmune atrophic gastritis was found; and achlorhydria was detected. All these findings met the diagnostic criteria of gastric NET type 1. The current diagnosis was: gastric NET type 1 (G1, T2N0M0, phase IIA); rectal NET (G1, T2N1M0, phase IIIB). The recommended treatment protocol was: endoscopic resection + sandostatin + traditional Chinese medicine (TCM) treatment. After the larger gastric lesions were resected under endoscope, the multiple small lesions that could not be completely resected were treated with sandostatin and TCM medicinals. The sandostatin treatment lasted one year and then stopped for 6 months, while the TCM medicinals were constantly used. Gastroscopy was performed every 6 months and colonoscopy every 12 months.
Treatment and follow-up
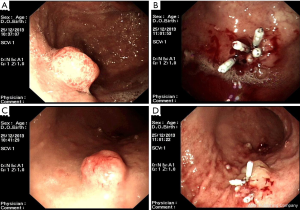
On December 25, 2013, endoscopic resection (Figure 6) was performed to remove a polyp sized 1.5 cm in the fundus of stomach; 12 smaller lesions sized 0.1–1.2 cm in the gastric body were removed using electrotomy. Pathology: in the fundus of stomach: NET (G1) (mitotic figure, <2/10 HPF; Ki-67, +2%). Immunohistochemistry: CD56 (focally +), CgA (+), Syn (+), and Ki-67 (+2%). In the body of stomach: NET (G1) (mitotic figure, <2/10 HPF; Ki-67, +2%). Immunohistochemistry: CD56 (focally +), CgA (+), Syn (+), and Ki-67 (+2%).
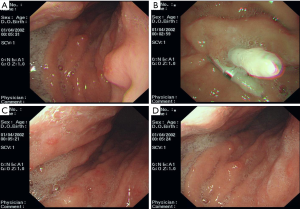
On February 20, 2014, gastroscopy (Figure 7) revealed multiple polyps in the fundus of stomach, with the largest one being 0.6 cm in diameter. These polyps were removed by clamping for biopsy. Multiple polyps were also seen in the body of stomach, with the largest one being 0.4 cm in diameter. Pathology: In the fundus of stomach: NET (G1) (mitotic figure, <1/10 HPF). Immunohistochemistry: CD56 (+), CgA (+), Syn (+), and Ki-67 (+2%). In the body of stomach: moderate chronic inflammation was seen in the mucosa, along with decreased proper glands and thickening of muscularis mucosae; also, nested cells were found in the proper layer. Based on immunohistochemical findings, neuroendocrine hyperplasia was considered. Since the specimens were relatively small and obtained from localized sites, the possibility of NET (G1) could not be ruled out. The immunohistochemical findings included: CD56 (+), CgA (+), Syn (+), and Ki-67 (−).
She began to receive sandostatin treatment (20 mg q28d) on February 28, 2014, which lasted 15 months (till May 22, 2015).
On November 3, 2014, gastroscopy and pathology were performed. A little clear liquid was seen in the mucus pool in the fundus of stomach, and a titanium clip was visible. Also, 4–5 polypoid lesions sized 0.15–0.40 cm were found in the upper and middle portions of the greater curvature of the stomach; four of them were removed for pathology. The antral mucosa was coarse. Two specimens were obtained from the gastric antrum. Also, two specimens were obtained from the greater curvature of the lower gastric body. The pathological diagnosis was gastric body NET (G1).
On April 21, 2015, gastroscopy (Figure 8) and pathology were performed. A little clear liquid was seen in the mucus pool in the fundus of stomach, and a titanium clip was visible. The mucosa of the greater curvature of the gastric body was coarse. Also, the antral mucosa was coarse. Two specimens were obtained from gastric antrum, gastric body, and gastric fundus, respectively. Pathology: neuroendocrine cell proliferation in gastric fundus and gastric body; no NET was found.
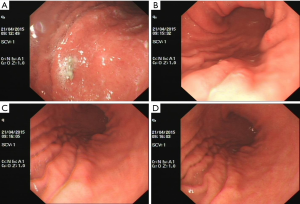
The final sandostatin treatment was carried out on May 22, 2015; 7 months later, gastroscopy (Figure 9) and pathology were performed on December 17, 2015. A little clear liquid was seen in the mucus pool in the fundus of stomach. Multiple tiny polyp-like uplifts were seen in the greater curvature of the gastric body. Four biopsy specimens were obtained. The antral mucosa was coarse. Two biopsy specimens were obtained. Pathology: gastric antrum: mild chronic mucosal inflammation, hyperplasia of muscularis mucosa, and linear proliferation of neuroendocrine cells; gastric body: atrophic gastric mucosa, intestinal metaplasia, and hyperplasia of muscularis mucosa, along with micronodular proliferation and focal dysplasia of neuroendocrine cells. Immunohistochemical staining showed focal dysplasia of neuroendocrine cells (<0.1 mm in diameter), which were located on the proliferation belt of mucous neck cells. Thus, the possibility of improper sampling of NET (G1) could not be ruled out. Immunohistochemistry: CgA (+), Syn (+), and CD56 (+).
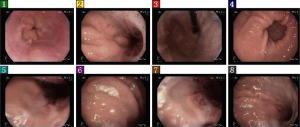
She re-initiated sandostatin treatment on December 28, 2015 till today. From 2014 to December 2015, abdominal MRI and pelvic CT were performed every 6 months, which did not show any metastasis.
Treatment summary
In December 2013, endoscopic resection was performed to remove a polyp sized 1.5 cm in the fundus of stomach; 12 smaller lesions sized 0.1–1.2 cm in the gastric body were removed using electrotomy.
In February 2014, gastroscopy revealed multiple polyps in the fundus of stomach, with the largest one being 0.6 cm in diameter. These polyps were removed by clamping for biopsy. Multiple polyps were also seen in the body of stomach, with the largest one being 0.4 cm in diameter.
In February 2014, treatment with sandostatin and TCM medicinals was initiated.
In November 2014, gastroscopy showed the decrease in the number of polyps in gastric fundus and gastric body and the pathological diagnosis was NET (G1).
In April 2015, gastroscopy showed that there was no obvious tumor in gastric fundus or gastric body; pathology showed the proliferation of neuroendocrine cells but did not find NET.
From June to December 2015, sandostatin was stopped and only TCM medicinals were used.
In December 2015, gastroscopy showed multiple tiny polyp-like uplifts in the greater curvature of the gastric body. The pathological diagnosis was dysplasia of neuroendocrine cells.
In December 2015, sandostatin treatment was re-initiated, and the TCM treatment continued.
Discussion
Diagnosis of gastric NET type 1
The typical clinical manifestations of gastric NET type 1 include fullness after meals, belching, or epigastric discomfort/pain; no heartburn/acid reflux or diarrhea may exist, although constipation is common. Fatigue may exist in patients with accompanying anemia. In some patients, there is no obvious gastric discomfort, whereas the condition is often found during routine gastroscopy. When endoscopy finds a lesion, which is confirmed by pathology as a gastric NET, further examinations should be performed for typing.
The well-differentiated gastric NETs can be classified into three types. The prognosis and treatment decision are completely different for different types. Type 1 gastric NET: it is associated with autoimmune atrophic gastritis, with elevated serum gastrin and achlorhydria; type 2 gastric NET: as a relatively rare type, it is associated with gastrinoma/MEN-1, with elevated serum gastrin and excessive gastric acid; and type 3 gastric NET: it is not associated with any disease, with normal gastrin and normal gastric acid secretion.
In our current case, gastroscopy showed multiple small polyps (up to 1.5 cm) in gastric fundus and gastric body. The pathological diagnosis was gastric NET (G1). The patient had no symptom such as heartburn/acid reflux and diarrhea. Thus, a type 1 gastric NET was suspected. Further laboratory tests showed elevated serum gastrin, positive gastric parietal cell antibody, and vitamin B12 deficiency; 24-hour gasric acid monitoring showed achlorhydria; gastroscopy revealed atrophic body gastritis; and gastric mucosal biopsy also showed atrophic body gastritis. Therefore, in this patient the gastric NET was associated with autoimmune atrophic gastritis and thus belonged to type 1.
The recurrence rate of type 1 gastric NET
The type 1 gastric NET has good prognosis and rarely results in metastasis. Currently most international guidelines recommend endoscopic treatment + endoscopic follow-up. For endoscopic resection, most authors recommend the application of endoscopic mucosal resection (EMR) for lesions sized 0.5 cm or more; for lesions smaller than 0.5 cm, watchful follow-up is recommended. About 80% of patients with type 1 gastric NET had multiple polypoid uplifts under gastroscope. The number of these multiple lesions can be 2 or more, 10 or more, or even dozens or more. A single lesion may recur after endoscopic resection, which may because the environments (atrophic gastritis, achlorhydria, and hypergastrinemia) causing gastric NET has not been changed. For 10 or more (and even dozens or more) small lesions, they can not be thoroughly resected under endoscope; thus, recurrence is even more common. Regular follow-up (generally, every 6–12 months) is important.
Role of somatostatin analogs in the treatment of type 1 gastric NET
During the pathogenesis of type 1 gastric NET, autoimmune atrophic gastritis and achlorhydria lead to antral G-cell hyperplasia and elevated serum gastrin, which stimulate the proliferation of ECL cells in gastric fundus and gastric body, atypical hyperplasia, and NET. Somatostatin analogues (SSA) can inhibit the secretion of gastric antral G cells and lower serum gastrin level and thus is effective in reducing gastric NET relapse. However, long-term use of SSA is needed; long-term withdrawal of this drug can resulted in the recurrence of gastric lesions. Since the type 1 gastric NET has good prognosis and the patients can often achieve long-term survival, SSA is not recommended for all type 1 gastric NET patients, in particular if the disease is still in its earlier stages. However, if the gastric lesions are small and multiple and cannot be resected endoscopically or if the lesions are small in number but repeatedly recur after endoscopic resection, SSA (e.g., sandostatin or lanreotide) treatment can be considered. Long-term and intermittent use is recommended (for instance, the drug may be used for 1 year and then stops for half a year). In our current case, the patient had multiple gastric lesions (more than 20). On December 25, 2013, totally 13 relatively larger lesions were resected endoscopically, leaving multiple small lesions. Two months later, gastroscopy showed multiple small polyps in gastric fundus and gastric body, with the largest one being 0.6 cm in diameter. These lesions were resected for pathology. Sandostatin treatment was used for 15 consecutive months and then stopped for 7 months. She re-initiated sandostatin treatment on December 16, 2015 till today, during which gastroscopy was repeatedly performed. The final gastroscopy was performed on December 17, 2015. During the 24-month follow-up, there was no recurrence of gastric lesion or systemic metastasis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Cite this article as: Dou D, Qiu X, Tan H. Gastric neuroendocrine tumor (NET): report of one case. Transl Gastroenterol Hepatol 2016;1:85.