Multimodality therapy for a case of neuroendocrine tumor in the hilum with multiple hepatic metastases
Brief disease history
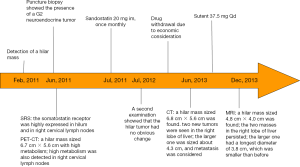
A 45-year-old male patient was admitted to the department of surgery due to “confirmed hilar neuroendocrine tumor for 30 months and liver metastasis for 4 months”. The patient had a history of hypertension for over 10 years. In recent years his blood pressure remarkably increased and had become difficult to control. Physical examination showed a palpable mass in right upper abdomen. The mass was about 4-cm in diameter, with firm texture and moderate activity. The disease course is showed in Figure 1.
Auxiliary examination
Positron emission tomography (PET)-CT (May 26, 2011)
A hilar tumor, with increased metabolism. A malignancy was considered. SUVmax 18.7. A nodule was located deep in the right upper side of the neck, along with increase metabolism. A malignancy was highly possible. Biopsy was required (biopsy was performed on August 1, 2014, which showed that the lesion was inflammation). PET-CT images were showed in Figure 2.
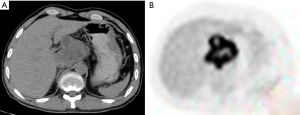
Contrast-enhanced CT (June 8, 2012)
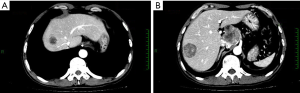
An irregular hilar mass extended towards the peritoneum and had poor borders with the caudate lobe of liver, left lobe of liver, and pancreatic head. It was adjacent to the inferior vena cava and the celiac trunk and sized 6.8 cm × 5.6 cm, showing heterogeneous enhancement. The size was similar to that in previous imaging. CT images were showed in Figure 3.
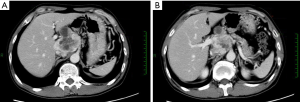
Contrast-enhanced CT (June 29, 2013)
An irregular hilar mass sized 6.8 cm × 5.6 cm, which was similar to that in previous imaging. In the right lobe of liver there were two new masses with blurred margins. The larger one had a longest diameter of 4.3 cm. Metastases were considered. CT images were showed in Figure 4.
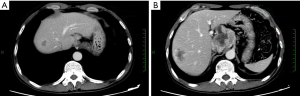
Contrast-enhanced CT (August 22, 2013)
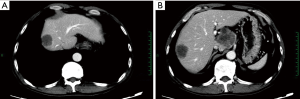
An irregular hilar mass sized 6.0 cm × 4.9 cm, which was slightly smaller than that in previous imaging. The two masses in the right lobe of liver persisted; the larger one had a longest diameter of 3.8 cm, which was also smaller than before. CT images were showed in Figure 5.
Contrast-enhanced CT (October 29, 2013)
An irregular hilar mass sized 4.9 cm × 4.9 cm, which was similar to that in previous imaging. Among the two masses in the right lobe of liver, the larger one had a longest diameter of about 3.9 cm; although it more rounded than before, the degree of enhancement decreased. CT images were showed in Figure 6.
MRI (December 11, 2013)
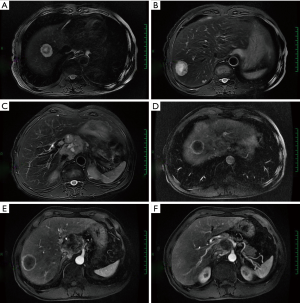
A fusion mass in the hilar area and the head of pancreas. It was sized 4.0 cm × 4.8 cm, with blurred margin and heterogeneous enhancement. A malignancy was considered. Multiple nodules and tumors were seen in the liver. The largest one was sized about 2.4 cm × 3.4 cm, with blurred margin and circular enhancement. Multiple metastases were considered. MRI were showed in Figure 7.
Based on the above findings, the following diagnoses were made: a hilar neuroendocrine tumor with multiple liver metastases; and, hypertension. As shown in the imaging, although there were liver metastases, the tumor was still believed to be resectable. The patient underwent surgical operation on January 6, 2014. Intraoperative exploration showed a hilar tumor sized 8.0 cm × 10.0 cm. The main body of the tumor was located on the left side of the hepatoduodenal ligament, whereas part of the tumor passed through the back side of the hepatoduodenal ligament to reach the right side of common bile duct. The upper border of the tumor was closely attached to the liver surface, invading the inferior vena cava; its inferior margin surrounded the common hepatic artery; and, its left margin reached the left side of the left gastric vessel.
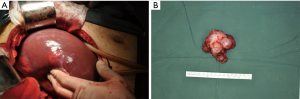
Multiple liver metastases were seen. The largest one was located in hepatic segment VIII and at the border between segment VII and VIII, with a diameter of about 5 cm. Multiple nodules (about 0.5 cm in diameter) were observed on liver surface. Intraoperative photos were showed in Figure 8. No metastasis to other organ or abdominal and pelvic implantation metastasis was observed. The surgical procedure performed was resection of hilar tumor and multiple liver metastases. Compression of the tumor during the surgery caused the fluctuation of blood pressure, which ranged between 200/100 and 70/40 mmHg. The heart rate fluctuated between 130 and 70 beats/min. Pheochromocytoma was highly suspected. Noradrenaline was applied to maintain blood pressure before the transection of tumor vessels. The blood loss was 800 mL. Thus, 400 mL of red cell suspension and 200 mL of plasma were tranfused.
After the surgery, norepinephrine (NE) was pumped to increase blood pressure following initial volume expansion (IVE) and rehydration. The recovery was smooth.
Post-operative pathology
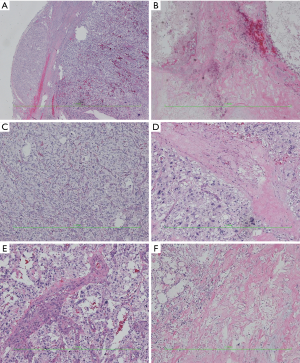
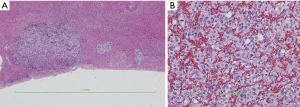
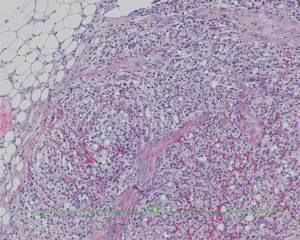
Pathological diagnosis: based on the morphology, immunohistochemical findings, and clinical manifestations, a diagnosis of “malignant extra-adrenal paraganglioma” was made. Some tumor cells showed mild degeneration, along with fibrosis, which was consistent with the features of mild postoperative changes.
Tumor involvements included nodules in lesser sac, hepatic nodules in segment VI, small hepatic nodules in segment VI, small hepatic nodules in segment V, hepatic nodules in segment VIIII, mass at the border between segments VII and VIII, mass in the bare area of liver, and hepatic mass in segment VIII.
No cancer was seen in the gallbladder tissues.
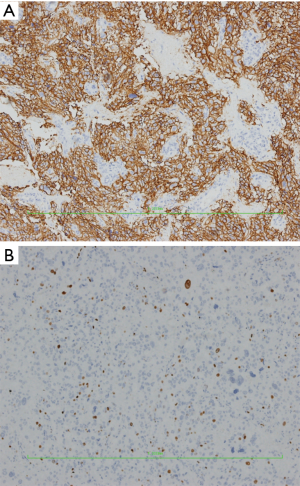
Hematoxylin-eosin (HE) and immunohistochemistry (IHC) staining
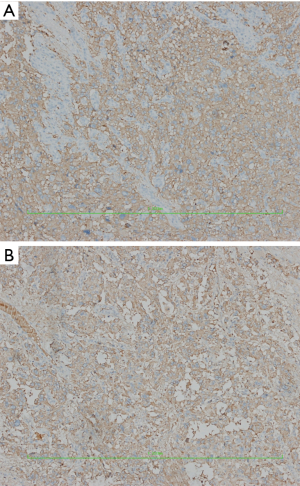
IHC showed: CD56(3+), chromogranin A(2+), synaptophysin(2+), CK18(−), CK19(−), CK20(−), CK7(−), hepatocyte(−), CK8(2+), EMA(−), GFAP(−), S100[+(stroma)], and Ki-67 (10%+).
HE staining pictures were showed in Figures 9-11. IHC staining pictures were showed in Figures 12,13.
Case discussion
Classification and nomenclature of paraganglioma
In 1912, Pick recommended that intra-adrenal chromaffin tumors be called pheochromocytomas and that all extra-adrenal chromaffin tumors be termed paragangliomas, which were also known as nonchromaffin paraganglioma or chemodectoma. Traditionally it was believed that paragangliomas accounted for only 10–15% of all pheochromocytomas. This proportion has risen in recent years to about 20%. As an extremely rare neuroendocrine tumor in soft tissues, paragangliomas belong to amine precursor uptake and decarboxylation (APUD) tumors. They arise from neural crest cells and can synthesize, store, and secret catecholamines (CAs), thus producing a variety of peptide neurohormones and chromaffin granules. In 2004, the Word Health Organization (WHO) defined the pheochromocytoma (or, adrenal paraganglioma) as tumors arising from CA-producing adrenal medullary chromaffin cells. In contrast, tumors arising from sympathetic and parasympathetic ganglions were defined as extra-adrenal paragangliomas. It has been increasingly recognized that the pheochromocytoma specifically refers to adrenal pheochromocytoma, whereas the traditionally ectopic or extra-adrenal pheochromocytomas are collectively termed as paragangliomas.
Based on the primary sites, the extra-adrenal pheochromocytomas can be further divided into four types: (I) arising from visceral arch: it is related with the development of visceral arch and is also known as chemodectoma (e.g., carotid chemodectoma or aortic body chemodectoma); (II) type of vagus nerve: this type is distributed in the locations of peripheral vagus nerve; (III) arising from the aortic sympathetic ganglia: this type is mainly distributed in segmental ganglia in the neck, chest, abdomen, and retroperitoneum; and (IV) visceral type: this type is mainly related with vascular organs such as atria, atrial septum, urinary tract, and liver. Most extra-adrenal paragangliomas are located near the posterior abdominal wall and the abdominal aorta (from the upper abdomen till the bottom of the pelvis), mainly arising from the Zuckerkandl’s body in this region.
Based on the clinical manifestations and blood CA level, paragangliomas can be divided into functional and non-functional types, among which the functional paragangliomas account for 10–20%, with the main manifestations including high blood pressure, heart palpitations, and elevated blood CA levels.
Differences benign and malignant paragangliomas
Typically paragangliomas are rare and benign tumors, but about 10% cases may have malignant potential. It has been widely believed that paragangliomas are slowly growing tumors with potentially or low-grade malignant behaviors. Some authors tried to predict the potential malignancy from a morphological perspective including tumor size, nuclear pleomorphism, mitotic figure, necrosis, and Ki-67 index, but with unsatisfactory results. Research has found that the pathological findings of a paraganglioma were not necessarily parallel with its biological behaviors. A morphologically “benign” paraganglioma can also become metastatic. The histological feature alone is not sufficient to distinguish benign from malignant paragangliomas. The diagnosis of a malignant paraganglioma relies mainly on relapse and metastasis. Lymph node metastasis is the most reliable indicator for differentiation. The value of capsular and/or vascular invasion is limited. A malignancy may be diagnosed if there is extensive capsular involvement or if the tumor invades the surrounding soft tissues. Malignant paraganglioma may spread to the lung, liver, kidney, brain, and lymph node via blood stream and/or lymphatic system.
Liver metastases occurred in our current case. A malignancy could be diagnosed according to its biological behaviors. Although preoperative assessment showed that surgery in this patient might be difficult and risky, aggressive surgery should be carried out to remove the tumor.
Special examinations for clinically suspicious pheochromocytoma or paraganglioma
There is an estimated preoperative misdiagnosis rate of 100% for pheochromocytoma or paraganglioma. Further understanding and knowledge about this disease may facilitate the correct diagnosis of this disease.
The following examinations may be applied for clinically suspicious pheochromocytoma or paraganglioma:
- Iodine-131 metaiodobenzylguanidine (131I-IMBG): 131I-IMBG has a high affinity with chromaffin cell tissues. It can be used for both qualitative diagnosis and locating. It is more accurate than CT in locating multiple maligant, and/or metastatic pheochromocytoma. It is particularly valuable for non-functioning pheochromocytomas;
- Octreotide (OCT) scan: somatostatin-receptor (SSTR) imaging using the radioactive-labeled OCT tracers can be useful for locating endocrine tumors and help to judge the efficacy of somatostatin treatment. While IMBG is more valuable for adrenal pheochromocytoma, OCT is superior in diagnosing paraganglioma. They can complement each other. IMBG is preferred for patients with clinical manifestations of a chromaffin cell tumor, and OCT should be further applied in patients with negative IMBG findings;
- 18F-fluorodeoxyglucose positron emission tomography (PET): Literature has demonstrated that PET can successfully display and accurately locate pheochromocytomas.
Treatment of paraganglioma
Paraganglioma is not sensitive to radiotherapy or chemotherapy. Early or complete surgical resection remains the mainstream treatment for it. Even if the tumor has already become large or if there is a relapse, surgery should always be considered if the patient can tolerate it. It has been reported that the surgical resection could reach 75.5%. Resection of the tumors should be thorough and complete to prevent relapse and increase survival rate. For patients with suspected malignant paraganglioma, routine follow-up visits should be arranged after surgery; prophylactic radiotherapy may be applied if necessary. The paragangliomas usually have a good prognosis, with a 5-year survival rate of 33% and a 10-year survival rate of 25%.
Preoperative preparation for pheochromocytomas/paragangliomas
For functional pheochromocytomas/paragangliomas, adequate preoperative preparation is the key to the success of surgery. Without the routine administration of α-receptor blockers, the surgical mortality rate of pheochromocytomas patients can be as high as 24–50%; in contrast, the surgical mortality rate is below 3% after adequate preoperative preparation using drugs. The purpose of preoperative drug preparation include: (I) to block the effect of excessive CA, maintain the normal blood pressure and heart rate/rhythm, and improve the function of the heart and other organs; (II) to correct hypovolemia; (III) to prevent the fluctuating blood pressure due to the massive release of CA induced by surgery and anesthesia and reduce the occurrence of severe complications such as acute heart failure and pulmonary edema.
Controlling high blood pressure
- α-blockers (recommended): the most commonly used drug is the long-lasting non-selective α-blocker phenoxybenzamine (initial 5–10 mg bid; can be increased by 10–20 mg every 2–3 days). If the paroxysmal symptoms are controlled, the blood pressure is normal or slightly low, and/or there is postural hypotension or stuffy nose, the drug dose is appropriate (typically 30–60 mg or 1 mg/kg daily, orally administered 3–4 times per day, with a daily dose not exceeding 2 mg/kg. Alternately, α1-blockers can also be used. During the drug administration, increasing dietary salt intake may decrease the occurrence of orthostatic hypotension and facilitate volume expansion;
- Calcium-channel blockers (recommended): calcium-channel blockers can block the entry of NE-mediated calcium ions into the vascular smooth muscle cells, thus achieving the control of blood pressure and arrhythmia. Also, they can prevent CA-related coronary artery spasm and facilitate the improvement of cardiac function. They have comparable efficacy to α-blockers but do not cause orthostatic hypotension.
Calcium-channel blockers are recommended to replace α-receptor blockers in the following three conditions:
- Alpha-receptor blocker monotherapy cannot satisfactorily control blood pressure and a combination with calcium-channel blockers can improve the therapeutic effectiveness and reduce the dose of α-receptor blockers;
- The α-receptor blockers have severe side effects that cannot be tolerated by the patients;
- The blood pressure is normal or only intermittently increased, replacing the α-receptor blockers with calcium-channel blockers can avoid hypotension or orthostatic hypotension.
Controlling arrhythmias
For CA- or α-receptor blocker-mediated tachycardia (>100–120/min) or supraventricular arrhythmias, β-receptor blockers may be added to control the heart rate below 90/min. However, the β-blockers must be used 2–3 days after α-blockers because β-blocker monotherapy may block the effects of adrenaline in activating β2 receptor and dilating vessels and thus induce fatal complications including hypertensive crisis, myocardial infarction, and pulmonary edema. Cardioselective β2 blockers such as atenolol and metoprolol are recommended.
Treatment of hypertensive crisis
Sodium nitroprusside, phentolamine, or nicardipine via venous pump is recommended for the management of hypertensive crisis
Duration of preoperative drug preparation
An interval of at least 10–14 days is recommended, and an interval of 4–6 weeks for patients with frequent attacks. The preoperative drug preparation is regarded as adequate in the following conditions:
- The blood pressure is maintained at 120/80 mmHg, with a heart rate
- Without paroxysmal high blood pressure, heart palpitations, or sweating;
- With increased body weight and hematocrit
- With mild nasal congestion, lost feeling of coldness in the extremities, experiencing warm feeling in the extremities, and/or ruddy nail bed (which suggests good microcirculation perfusion).
Good communication between surgeon and anesthesiologists is required before surgery. In our current case, the fact that the tumor was functional was not considered before surgery, and the patient experienced fluctuations of heart rate and blood pressure during the surgery. Fortunately, the anesthesiologists dealt with the situation timely and properly, thus avoiding a perioperative death.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Cite this article as: Li Y, Huang Z, Zhang Y, Zhao H. Multimodality therapy for a case of neuroendocrine tumor in the hilum with multiple hepatic metastases. Transl Gastroenterol Hepatol 2016;1:81.