Targeting angiogenesis in gastrointestinal tumors: current challenges
Introduction
Targeted therapy has become a part of the spectrum in treatment of various cancers and colorectal cancer (CRC) is no exception, especially in the setting of metastasis. The introduction of the drugs that target the angiogenic pathway has contributed to the decrease in mortality rates of CRC and proved beneficial to the patients. As per cancer statistics 2015, the number of newly diagnosed cases in CRC would be 132,700 and projected 49,700 deaths (1). CRC remains the third leading cause of mortality despite advances in treatment. In this review along with discussing about the angiogenesis inhibitors we also discuss about the challenges in terms of resistance and biomarkers.
Basis of anti-angiogenic therapy
Angiogenesis is defined as formation of new blood vessels from the pre-existing ones. It is essential for the progression and growth of cancer. Presence of adequate blood supply is very essential for the tumor cells to grow and metastasize (2). An “angiogenic switch” occurs turning these tumor cells into an invasive phenotype, also called as angiogenic phenotype. Tumor cells characterized by angiogenic phenotype have potential to release pro-angiogenic growth factors. As in normal adult tissues, tumor cells maintain a balance between the pro-angiogenic and the anti-angiogenic growth factors. But in some tumor cells, the balance is lost and the pro-angiogenic properties take over stimulating endothelial proliferation, neovascularization leading to tumor growth and metastases (3,4). Hence, inhibiting tumor angiogenesis has become a target for treatment of cancers. In 1971, Judah Folkman was the first to postulate the idea of developing angiogenesis inhibitors in the treatment of human cancer (4).
One of the pro-angiogenic factors that have been studied well is the vascular endothelial growth factor (VEGF). The VEGF/VEGF-Receptor pathway is a key factor in promoting tumor angiogenesis (5). VEGF family contains 6 glycoproteins, namely VEGF A, B, C, D, E and placental growth factor. The most important glycoprotein of the whole family is VEGF-A. VEGF-A contains 121 amino acids and weighs about 45kDA and was first described by Senger et al. (6). Leung et al. later isolated and cloned VEGF-A as an endothelial specific mitogen (7).
The VEGF binds to VEGF receptors, which exhibit tyrosine kinase activity. The three types of receptors are VEGFR1 or Flt-1 and VEGFR2 or KDR/Flk-1, VEGFR3. The important receptor amongst the three is the VEGFR2 (8). The kinase activity of VEGFR1 is low, but the affinity for VEGF is high. VEGFR3 has limited role in vascular angiogenesis (9).
The regulators of the VEGF/VEGFR pathway are many. To name, the factors which increase the expression of VEGF are tissue hypoxia via the hypoxia inducing factor (HIF), growth factors like epidermal growth factor receptor (EGFR), insulin like growth factor (ILGF), oncogenes like Ras, Src and tumor suppressor genes like P53, PTEN, VHL (10-13). There are two hypoxia-inducing factors namely HIF1A and HIF2A. During hypoxic conditions, there is increased expression of HIF1A, leading to the increased expression of downstream pro-angiogenic factors like VEGF. To cite an example, in clear cell renal carcinoma, there is inactivation of von Hippel Lindau gene, leading to increased expression of HIF1A, which subsequently up-regulates the VEGF pathway. Hence, anti-VEGF therapies are promising in renal cell carcinoma (14).
VEGF increases the permeability of the post capillary venules, leading to plasma protein leakage into the extra cellular matrix, leading to leakage of the fibrinogen, which is converted to fibrin. This stimulates the signaling pathways that promote migration and proliferation of the endothelial cells (15). VEGF pathway has increased expression in most human cancers, thereby making it a potential target for anti-angiogenic drugs. There are different ways of blocking the VEGF pathway and hence controlling tumor angiogenesis. The important ones are, blocking the interaction of the VEGF to its receptor, affecting the VEGF ligand binding, blocking the intracellular function of the VEGF signal and decreasing the production of pro-angiogenic factors (16).
The other pro-angiogenic growth factors playing a role in angiogenesis have also been reported. They are fibroblast growth factor (FGF), platelet derived endothelial cell growth factor, interleukin-8, angiogenin, transforming growth factor alpha and beta. However, the most important angiogenic growth factor in CRC is VEGF (17).
Anti-angiogenesis in CRC
Anti-angiogenic therapy has been approved for cancers like metastatic colorectal cancer (mCRC), metastatic renal cell cancer, non-small cell lung cancer (NSCLC), metastatic gastric cancers, glioblastoma multiforme, hepatocellular carcinoma and recurrent/metastatic cervical cancer. The molecular pathways that are targeted in CRC are VEGF and EGFR. Initially, cytotoxic chemotherapy has been the standard of care, but later on addition of anti-angiogenic therapy has increased overall survival (OS) in the metastatic setting. Studies in the adjuvant setting have failed to demonstrate a benefit (18). Here we describe the details of bevacizumab, aflibercept, regorafenib, ramucirumab and other novel drugs in CRC.
Bevacizumab
Bevacizumab is an immunoglobulin G1 monoclonal antibody against the VEGF-A ligand and the first anti-angiogenic drug approved by FDA in combination with cytotoxic chemotherapy in first line setting of mCRC patients. The approval by FDA was based on a pivotal phase III trial AVF2107 which showed increased response rates, progression free survival (PFS) and OS. Eight hundred thirteen patients with untreated metastatic CRC were included of which 402 patients received irinotecan, bolus 5-FU/LV (IFL) plus bevacizumab (5 mg/kg every 2 weeks) and 411 receiving IFL plus placebo. Results of the bevacizumab arm were favorable in comparison to the placebo arm as reflected in the median OS (20.3 vs. 15.6 months; HR 0.66; P<0.001), PFS (10.6 vs. 6.2 months; HR 0.54; P<0.001), response rates (44.8% vs. 34.8%; P=0.004) and durable responses (10.4 vs. 7.1 months; P=0.001) (19).
Prior to the AVF2107 pivotal trial, there were two phase II trials done by Kabbinavir et al. demonstrated that the addition of bevacizumab increased the time to disease progression. In the first study published in 2003, 104 untreated metastatic patients were enrolled and were divided into three groups, group 1 which only received 5-FU (500 mg/m2)/LV (500 mg/m2), group 2 received a low dose bevacizumab (5 mg/kg every 2 weeks) plus 5-FU/LV, group three received a high dose bevacizumab (10 mg/kg every 2 weeks) plus 5-FU/LV. The addition of bevacizumab showed higher response rates in both the dose arms when compared to the control arm. Results for control arm vs. bevacizumab arm in response rates were 17% vs. 40% in low-dose group (P=0.29) vs. 24% in high-dose group (P=0.434), median time to disease progression 5.2 vs. 9.0 months in low-dose group (P=0.05) vs. 7.2 months in high-dose group (P=0.217) and median survival of 13.8 vs. 21.5 months in low-dose group vs. 16.1 months in high-dose group (20). This lead to the study of bevacizumab 5 mg/kg plus chemotherapy in the first line for mCRC published in 2005. In this study, 104 patients were enrolled and randomized to 5-FU/LV plus placebo and 5-FU/LV plus bevacizumab. Results for the bevacizumab vs. the placebo arm were median survival of 16.6 vs. 12.9 months (P=0.16), median progression-free survival of 9.2 vs. 5.5 months (P=0.0002), response rates of 26.0% vs. 15.2% (P=0.055) and duration of response was 9.2 vs. 6.8 months (P=0.088) (21,22).
Saltz et al. conducted a phase III trial to evaluate the efficacy of adding bevacizumab to oxaliplatin based combination chemotherapy regimens such as capecitabine plus oxaliplatin (XELOX) or fluorouracil/folinic acid plus oxaliplatin (FOLFOX-4). A total of 1,401 patients were randomly assigned XELOX and then to bevacizumab (7.5 mg/kg if given with XELOX, a 3-week cycle and 5 mg/kg if given with FOLFOX-4, a 2-week cycle) or placebo. A PFS advantage was seen in the bevacizumab containing arm (9.4 vs. 8 months; P=0.0023), however there was no statistically significant difference in median OS between the two groups (21.3 vs. 19.9 months; P=0.077). A failure to see a difference in the OS could have been due to bevacizumab not being continued beyond disease progression (23).
There are no randomized trials comparing FOLFIRI (5-fluorouracil, folinic acid, irinotecan) with and without bevacizumab to date. Petrelli et al. published a pooled analysis of 29 prospective and retrospective studies to evaluate the activity and efficacy of FOLFIRI plus bevacizumab in the front line setting of metastatic CRC patients. A total of 3,502 patients were studied. Pooled analysis of response rate was 51.4%, median PFS was 10.8 months (95% CI 8.9–12.8) and OS was 23.7 months (95% CI 18.1–31.6) in patients who received FOLFIRI plus bevacizumab. This study justified the use of FOLFIRI plus bevacizumab in the first line setting of untreated metastatic CRC patients (24).
To evaluate the efficacy of triplet chemotherapy regimen with bevacizumab, the TRIBE trial was conducted (TRIplet plus BEvacizumab). The TRIBE was a phase III randomized trial, in which 508 patients were randomly assigned to two groups, FOLFIRI with bevacizumab (control group) and FOLFOXIRI with bevacizumab (study group). The results of the study and control group were, median PFS of 12.1 and 9.7 months (95% CI 0.62–0.90; P=0.003), objective response rate 65% and 53% (P=0.006) respectively. Though the primary end point (PFS) was reached, patients in the study arm experienced higher incidence of toxicities like grade 3 or 4 stomatitis, diarrhea, neuropathy and neutropenia (25).
Cremolini et al. published an update of TRIBE study providing results of OS and treatment effect in the RAS and BRAF molecular subgroups. At a median follow-up of 48.1 months, median OS was 29.8 months (95% CI 26.0–34.3) in the FOLFOXIRI plus bevacizumab group compared with 25.8 months in the FOLFIRI plus bevacizumab group (95% CI 0.65–0.98; P=0.03). In the whole cohort median OS was 37.1 months (95% CI 29.7–42.7) in the RAS and BRAF wild-type subgroup compared with 25.6 months in the RAS-mutated (95% CI 1.11–1.99) and 13.4 months in the BRAF-mutated subgroup (95% CI 1.75–4.46; P<0.0001). In the subgroup of RAS and BRAF wild-type patients, those in the FOLFOXIRI plus bevacizumab group reported a median OS of 41.7 months (95% CI 30.1–53.1) compared with 33.5 months in the FOLFIRI plus bevacizumab group (HR 0.77; 95% CI 0.46–1.27). There was no statistically significant difference in the treatment effect in RAS and BRAF molecular subgroups (P=0.52) (26).
Bevacizumab can also be given in the second line setting of metastatic CRC patients based on the results of the Phase III E3200 study. Eight hundred twenty-nine mCRC patients previously treated with a fluoropyrimidine and irinotecan were randomly assigned to one of three treatment groups: oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) with bevacizumab, FOLFOX4 without bevacizumab and bevacizumab alone. The primary end point was OS, with other end points being progression-free survival, response rates. The median duration of survival for the group treated with FOLFOX4 and bevacizumab was 12.9 months compared with 10.8 months for the group treated with FOLFOX4 alone (HR =0.75; P=0.0011), and 10.2 months for those treated with bevacizumab alone. The median progression-free survival for the group treated with FOLFOX4 in combination with bevacizumab was 7.3 months, compared with 4.7 months for the group treated with FOLFOX4 alone (P<0.0001), and 2.7 months for those treated with bevacizumab alone. The overall response rates were 22.7%, 8.6%, and 3.3%, respectively (P<0.0001 for FOLFOX4 with bevacizumab vs. FOLFOX4) (27).
There are two studies that evaluated bevacizumab in the maintenance setting are MACRO TTD (Maintenance treatment in advanced CRC for the Treatment of Digestive Tumors) and CAIRO3. MACRO TTD, a phase III study had evaluated the role of bevacizumab alone in the maintenance setting. Four hundred and eighty patients after receiving induction therapy with capecitabine plus oxaliplatin (XELOX) with bevacizumab were randomized to bevacizumab alone and bevacizumab plus XELOX. The authors had set a pre-specified non-inferiority limit of hazard ratio for PFS at 1.32. After a median follow-up of 29 months median PFS in patients receiving maintenance XELOX with bevacizumab vs. bevacizumab alone was 10.4 and 9.7 months respectively (HR of 1.10; 95% CI 0.89–1.35). As the HR is >1.32, this study thus did not confirm non-inferiority of bevacizumab maintenance when compared to XELOX with bevacizumab (28). The CAIRO3 was conducted to evaluate the efficacy of maintenance treatment with capecitabine plus bevacizumab versus observation. Five hundred fifty-eight patients who had a stable disease after being treated with six 3-weekly cycles of capecitabine, oxaliplatin, and bevacizumab (CAPOX-B) were randomly assigned to maintenance treatment with capecitabine and bevacizumab or observation. After initial progression, patients in both the groups received maintenance treatment until second progression (PFS2), the primary end point of the study. After a median follow-up of 48 months PFS2 was 11.7 months in the maintenance group and 8.5 months in the observation group (95% CI 0.56–0.81; P<0.0001). Overall the maintenance treatment was tolerated well without affecting quality of life but incidence of hand foot skin reaction was higher in this group (23% of patients were affected) (29).
Observational and randomized data support the use of bevacizumab despite progression on a bevacizumab containing therapy. Survival rates of patients who received bevacizumab beyond progression (BBP) were reported in a large observational-study, the BRiTE study (Bevacizumab Regimens: Investigation of Treatment Effects and Safety). Of 1,953 patients, 1,445 patients who were enrolled in the study experienced progression of disease (PD). These patients were classified into three groups, no post-PD treatment (n=253), post-PD treatment without bevacizumab (no BBP; n=531), and BBP (n=642). Median OS was 12.6, 19.9 and 31.8 months in the no post-PD treatment, no-BBP, and BBP groups respectively. The BBP group has shown significantly improved survival when compared to no BBP group (HR, 0.48; P<0.001) (30). Another observation cohort study, the ARIES, also reported that bevacizumab given after first progression, had a higher median post progression survival for BBP (n=438) when compared to no BBP (n=667) reported as 14.4 vs. 10.6 months (HR 0.84; 95% CI 0.73–0.97) respectively (31).
ML18147, a phase III trial assessed the continued use of bevacizumab with second line chemotherapy after progressing on first line bevacizumab containing chemotherapy. Four hundred and nine patients were assigned to bevacizumab plus second line chemotherapy and 411 to chemotherapy alone. After a median follow-up of 11.1 months in the bevacizumab group and 9.6 months in the chemotherapy group median OS of 11.2 months (95% CI 10.4–12.2) for bevacizumab plus chemotherapy and 9.8 months for chemotherapy alone (HR 0.81; 95% CI 0.69–0.94; P=0.0062) was reported favoring the bevacizumab group (32). The BEBYP (The Bevacizumab Beyond Progression) trial also produced similar results and showed that there was a significantly higher PFS in patients who received BBP (6.8 vs. 5.0 months; HR 0.70; P=0.010) (33). Thus, we could conclude that bevacizumab can be administered in mCRC beyond progression as studies have shown improvement in OS and also PFS.
Bevacizumab is associated with an increased bleeding risk. It is not uncommon to see patients with malignancies to have a concurrent thrombotic risk requiring anticoagulation. It remains a concern whether to continue bevacizumab in the patients who are on anticoagulation. Leighl et al. published a report analyzing three randomized placebo controlled studies that permitted using therapeutic anti coagulation along with the bevacizumab or placebo. Two out of three studies included in this report were on mCRC (19,23). The authors concluded that severe bleeding event rates in patients with bevacizumab who were receiving anticoagulation were similar in frequency to the placebo groups, ranging from 0 to 8% or 0 to 67 events per 100 patient-years. Thus, there is some evidence to suggest that bevacizumab can be used safely in patients on anti coagulation (34,35).
Ziv-aflibercept
A fusion protein consisting of VEGF-binding portions from the extracellular domains of human VEGF receptors 1 and 2 fused to the Fc portion of the human immunoglobulin G1 (36). It has high affinity to VEGF-A, VEGF-B and placental growth factor thereby inhibits binding to their receptors (37). Ziv-aflibercept is FDA approved for use in patients with mCRC who progressed or failed oxaliplatin therapy and is used in combination with FOLFIRI. There are no head to head trials comparing bevacizumab to ziv-aflibercept (38).
The study that led to the approval of this drug was the VELOUR study. The VELOUR study was a Phase III randomized study in which 1,226 patients were assigned to two groups, 612 patients in the aflibercept (4 mg/kg intravenously) plus FOLFIRI group and 614 patients in the placebo plus FOLFIRI group. Three hundred seventy-three (30.4%) patients who received prior bevaciuzmab were also included. The median OS was 13.5 vs. 12.06 months in the aflibercept group vs. the placebo group respectively (HR 0.817; 95.34% CI 0.713–0.937; P=0.0032). The median PFS was 6.90 vs. 4.67months (HR 0.758; 95% CI 0.661–0.869; P≤0.0001) in the aflibercept group vs. the placebo group respectively, favoring the aflibercept arm (39).
Tang et al. conducted a phase II trial in patients with previously treated mCRC. Seventy-five patients were enrolled into two different cohorts, one being bevacizumab naïve and the other is patients who received prior bevacizumab therapy and were given single agent aflibercept. There was limited activity of aflibercept as a single agent in the patients who were previously treated (40). The AFFIRM study, was a phase II randomized study comparing modified (m) FOLFOX6 alone or in combination with aflibercept in the first line setting. The primary end point was PFS after a period of 12 months. The median PFS was 8.48 months (95% CI 7.89–9.92) for the aflibercept plus mFOLFOX6 arm and 8.77 months (95% CI 7.62–9.27) for the mFOLFOX6 arm. Patients in the study arm experienced more adverse effects like increased neuropathy, diarrhea and also VEGF related side effects like hypertension and thromboembolic events. So, this study concluded that there was no improvement in PFS and patients experienced more adverse events (41).
Regorafenib
Regorafenib is an oral anti-angiogenic drug. It is a multi-kinase inhibitor that inhibits kinases at the endothelium level like VEGFR1, VEGFR2, VEGFR3, TIE2 and also at the tumor microenvironment level like PDGFR (platelet derived growth factor receptor) and FGFR (fibroblast growth factor receptor). It also inhibits other kinases which promotes oncogenesis like c-KIT, RET, B-RAF (42).
After demonstrating efficacy in phase I studies (43,44), CORRECT trial, a phase III study was done which lead to the regorafanib approval. This trial included 760 patients with metastatic CRC who were previously treated and were randomized in a 2:1 fashion, to receive regorafenib plus best supportive care (BSC) or placebo plus BSC. Median survival was 6.4 months in the study arm and 5 months in the placebo arm (HR 0.77; 95% CI 0.64–0.94; P=0.0052). The PFS was 1.9 and 1.7 months is the study and placebo arm respectively (HR 0.49; P<0.0001). Toxicities like hypertension, hepatotoxicity and hand foot syndrome were seen in higher proportion in the study arm (45).
Ramucirumab
Ramucirumab is a humanized immunoglobulin G1, targeted towards VEGFR2 (46). In April 2015, ramucirumab received approval by FDA for mCRC that progressed during or after first-line treatment with bevacizumab in combination with second-line FOLFIRI. The RAISE study, a large randomized double-blind phase III study enrolled 1,072 patients into two groups. One group received 8 mg/kg intravenous ramucirumab plus FOLFIRI and the other received placebo with FOLFIRI every 2 weeks until disease progression, intolerable adverse events or death. Median OS was 13.3 vs. 11.7 months (HR 0.844; 95% CI 0.730–0.976; P=0.0219) and PFS of 5.7 vs. 4.5 months (HR 0.79; P=0.0005) for patients in the ramucirumab group versus the placebo group respectively (47).
Novel drugs
There are some novel anti-angiogenic drugs that are in various phases of development. To name a few, fruquintinib, famitinib, and nintedanib, tanibirumab, vanucizumab (48). We discuss a few of the agents below.
Famitinib
This drug inhibits multiple kinases like VEGFR2 and VEGFR3, PDGFR, stem cell factor receptor c-KIT, FMS-like tyrosine kinase-3 receptor (FLT3) and the proto-oncogene tyrosine-protein kinase inhibitor RET. In a randomized multicenter double blind, placebo-controlled, phase II trial from China 154 patients with advanced CRC who failed second or later-line treatments were randomized in a 2:1 ratio to receive either famitinib or placebo at 25 mg each day in each treatment cycle. The median PFS was 2.8 and 1.5 months (P=0.0034; HR =0.58), objective response rate was 2.02% and 0.00% (P=0.54) and the disease control rate was 57.58% and 30.91% (P=0.0023) in the treatment group and control group, respectively. The side effects were grade 1–2 hand foot syndrome, proteinuria, neutropenia, thrombocytopenia and hypertension (49). There is an ongoing phase III study in China, the aim of this study is to assess whether famitinib can improve OS compared with placebo in a total of 540 patients with CRC who failed at least two lines of standard chemotherapy. The estimated completion date is July 2017 (ClinicalTrials.gov identifier NCT02390947).
Nintedanib
This is an oral novel triple inhibitor, as it inhibits VEGF, FGFR and PDGF. Due to its triple inhibition, it is believed that it plays a role in compensatory angiogenesis and could overcome the resistance developed due to VEGF directed therapy (50). A phase I/II study was done in patients with metastatic CRC to evaluate nintedanib in the first line setting. Patients were randomized in a 2:1 fashion, to receive mFOLFOX6 plus nintedanib (150 mg bid of 200 mg bid) and mFOLFOX6 with bevacizumab. In the phase I part maximum tolerated dose was determined to be 200 mg bid, which was used in the phase II part. The safety profile of nintedanib was acceptable with a fewer reports of hypertension, bleeding, thromboembolic events. The side effects pertinent to the study arm are nausea, vomiting, diarrhea, decreased appetite, constipation and neutropenia. The discontinuation rate was also less due to the adverse events in the nintedanib arm when compared to the bevacizumab arm (51). Nintedanib is being evaluated in a phase III study, LUME-Colon 1. The study is ongoing but not recruiting patients because May 2016 was the date for final data collection. OS and PFS are the primary outcomes measured (ClinicalTrials.gov identifier: NCT02149108) (52).
Other oral anti-angiogenic agents like sorafenib, sunitinib, vandetanib, and vatalanib have been tested in metastatic CRC, but the results have not been promising.
Sorafenib
This is a multi-kinase inhibitor of several receptor tyrosine kinases including VEGFR2, VEGFR3, PDGR beta, c-KIT, FLT3, and tyrosine kinase colony-stimulating factor 1 receptor and also pathways including RAF, MEK, ERK. NEXIRI is a phase I/II trial done to evaluate the efficacy of combined sorafenib and irinotecan as second or later-line treatment of patients with KRAS-mutated mCRC. The disease control rate was 64.9% (95% CI 51–77%). Median PFS was 3.7 months (95% CI 3.2–4.7) and OS was 8.0 months (95% CI 4.8–9.7) (53). The RESPECT trial, a phase IIb study done to evaluate the addition of sorafenib to first-line modified FOLFOX6 mCRC. One hundred ninety-eight patients were enrolled. Median PFS for sorafenib plus mFOLFOX6 was 9.1 vs. 8.7 months for placebo plus mFOLFOX6 (HR =0.88; 95% CI 0.64–1.23; P=0.46). There was also no difference between both the arms for OS (54).
Sunitinib
Sunitinib is also a multi kinase inhibitor targeting VEGFR1, VEGFR2, VEGFR3, PDGFR alpha and beta was tested as a first line in mCRC patients in a Japanese study, but without any promising results. Common toxicities are change of skin color, cardiac events, mucositis and hand foot syndrome (55).
Vatalanib
Vatalanib is an oral drug that blocks all VEGFR tyrosine kinase mediated signaling. This was studied in two phase III trials both in front line and second line setting but failed to demonstrate any survival benefit. In one study, the groups were FOLFOX-4 plus vatalanib vs. FOLFOX4 plus placebo (2nd line) PFS was 5.6 vs. 4.2 months (HR, 0.83, P=0.013) and OS of 13.1 vs. 11 months (HR, 1.0, P=0.957) respectively. In the first line setting, groups were FOLFOX4 plus vatalanib vs. FOLFOX4 plus placebo. For the two groups PFS was OS were not significant (PFS 5.6 vs. 4.2 months, HR 0.83, P=0.013 and OS 13.1 vs. 11.1 months, HR 1.0, P=0.957) (56,57).
Toxicities of anti angiogenic therapy
The most common adverse effects of anti-angiogenic therapy are hypertension, proteinuria, thromboembolism, gastrointestinal perforation, posterior reversible encephalopathy syndrome, and cardiac events (58). Trials with bevacizumab showed grade 3 medically manageable hypertension, hemorrhage, gastrointestinal perforation, arterial thromboembolism, wound healing complications and proteinuria (1–2%) (59,60). Regorafenib toxicities include fatigue, skin rash, diarrhea, hand foot skin reactions and hepatotoxicity (61). Ramucirumab toxicities are neutropenia, hypertension, fatigue and diarrhea (47).
Challenges
VEGF targeted therapy has been well studied and its benefits lead to its approval in cancers, including mCRC. Unfortunately, the effect is short lived and patients fail VEGF targeted therapy. The reason for failure is not well known, but may include variable mechanisms of resistance to these agents. The mechanisms of resistance may depend on the tumor type, as different tumors might have variable response to VEGF directed therapy (62). The main mechanisms of resistance that has been studied include tumor hypoxia, stromal cells recruitment and formation of new blood vessels through compensatory mechanisms other than the VEGF pathway. These three mechanisms promote in tumor growth despite inhibition of VEGF (63).
Jain et al. introduced the idea of “normalization” of tumor blood vessels in the setting of VEGF inhibition. This normalization reduces the interstitial pressure in the tumor and increases the delivery of the anti-angiogenic agent to the tumor. But, for any reason if the interstitial pressure in the tumor is increased, the delivery of the agent is hampered (64).
Anti-angiogenic therapy causes tumor hypoxia. Hypoxia in the tumor environment, aids in recruiting different stromal cells, which aids in neovascularization, leading to resistance to therapy. The cell types involved are endothelial cells, myelocytes and lymphocytes. Each cell might have varied mechanisms in aiding resistance. In the case of endothelial cells there may be increased expression of multidrug resistance proteins like P-glycoprotein (65,66). Other mechanisms included are the alterations in the glycosylation receptors of the VEGF2 (67). Hypoxia also induces bone marrow derived cells, like myelocytes that aids in sustaining angiogenesis. Increased pericyte coverage in the blood vessels of the tumor protects the tumor, thereby protecting the tumor from the anti-angiogenic therapy (68,69).
When there is suppression or inhibition of VEGF in the tumor, there is an increase in the other angiogenesis growth factors like FGF as a compensatory mechanism, thereby promoting angiogenesis. Hanahan et al. treated mice with a monoclonal antibody blocking VEGFR. The angiogenic inhibition was noted to be transient (10–14 days) and was followed by tumor regrowth with dense vasculature. These relapsed tumors showed increased levels of mRNA for FGF FGF like FGF1, FGF2 in mice (70). To prove the hypothesis that FGF promotes angiogenesis, mice were first treated VEGF inhibitor and later were treated with FGF trap to suppress FGF mediated vascularization. They observed that this combination slowed tumor growth and also decreased the re-vascularization (71).
NOTCH signaling pathway mediation has also been thought as a mechanism of resistance for anti-angiogenic therapy. Delta like ligand4 (Dll4) is a NOTCH ligand that is upregulated in the setting of hypoxia and by VEGF. This Dll4 aids in angiogenesis. Dll4 is only present in the endothelial cells of the tumor microenvironment. Xenograft models have shown that inhibiting Dll4-Notch pathway may overcome resistance to anti-VEGF therapy (72-74). Despite these efforts, resistance to anti-angiogenic therapy still remains as a major clinical challenge.
Biomarkers
Biomarkers could be predictive or prognostic. Presently, there are no biomarkers to predict the response to anti- angiogenic therapy. Hypertension, circulating levels of VEGF, expression of VEGF in the tumor cells, various imaging studies, single nucleotide polymorphisms, mismatch repair deficient tumors are some of the biomarkers that have been studied but not been used in daily clinical practice (75).
Hypertension caused by VEGF antibodies has been shown in different studies as a biomarker for positive outcome. Studies have shown that hypertension has been associated with high response rates, improved PFS and OS in patients with mCRC. In one single center retrospective study, 101 patients who received bevacizumab plus standard chemotherapy were studied. Blood pressure was measured prior to each infusion of bevacizumab. Fifty-seven patients (56%) developed ≥ grade 1 hypertension (HTN) and 44 (44%) remained normotensive during the study period. Overall response rate, PFS, OS in patients who developed HTN vs. normotensive patients was 30% vs. 20%; P=0.025, 10.5 vs. 5.3 months; P=0.008, 25.8 vs. 11.7 months; P<0.001 respectively (76). There are other retrospective studies that reproduced similar results and thus showing that bevacizumab-induced hypertension may be a prognostic factor for clinical outcome in advanced CRC patients (77,78).
The correlation between circulating VEGF levels and the outcome of anti-angiogenic therapy has been evaluated in many studies. Jubb et al. evaluated the tissue specimens from the patients included in the AVF 2107 trial. Three hundred twelve samples were collected and VEGF expression was assessed by in situ hybridization and immunohistochemistry on the available tissue. The levels of the VEGF did not predict the outcome (79). A meta-analysis of 20 studies has been done to assess the impact of VEGF on the OS and PFS in mCRC patients. The authors reported that high VEGF levels correlated to unfavorable survival (OS: HR =1.98, 95% CI 1.30–3.02; disease free survival: HR =2.10, 95% CI 1.26–3.49) and a 4.22-fold increase in the rate of distant metastases (80). As the results of the studies had been inconsistent it is believed that circulating VEGF levels can be a prognostic biomarker.
Imaging studies like contrast enhanced perfusion MRI and CT scans may give us an estimate of angiogenesis. But response to therapy can be seen in modern imaging techniques like nuclear PET that uses magnetic nanoparticles that targets avb3 integrin, which helps in targeting angiogenesis. Newer ultrasound techniques using gas filled microbubbles that target particular receptors of the endothelial cells are also being studied (81).
In the CONFIRM 1 and 2 trials, which studied vatalanib in mCRC, response has been related directly to mRNA levels of VEGFR1, LDH-A and GLUT1, but indirectly related to HIF1. It has to be noted that the studies involving vatalanib did not prove it to be superior, so it remains unclear if these results of the mRNA’s are to be taken to next level. Cytokines like IL-1β, IL-6, IL-8, stromal-cell-derived factor-1α may also act as pro- angiogenic growth factors and can be elevated in during treatment and may play a role as predictive biomarkers (82).
Most recently Suenaga et al. showed that serum levels of chemokine ligand 5 (CCL5) and VEGF-A levels could be markers for prediction of response in patients receiving regorafenib monotherapy. Out of all the examined markers, CCL5 levels less than the cut off value prior to starting therapy and decreasing VEGF A levels at the end of 21 days has proven to be effective as surrogates. These results were associated with better PFS (P=0.036) and good tumor shrinkage (P=0.021) (83).
Though there are various studies done in exploring predictive and prognostic biomarkers, challenges prevail in relating them to routine clinical practice.
Future perspectives
Anti-angiogenic therapy as a single agent or in combination with cytotoxic chemotherapy has its challenges. It is time to overcome those challenges. Focus is being shifted to combining immunotherapy with targeted therapies like anti-angiogenic agents, combining Dll4 inhibitors with VEGF inhibitors, discovering inhibitors for other pro-angiogenic growth factors like FGF and also against HIFA. Mechanisms to prevent resistance should also be studied. Some studies now are also being focused on vaccination strategies targeting the tumor endothelial cells. However, these are in the pre-clinical stages and further studies to be pursued to promote the bench to bedside approach (68).
The exciting concept of immunotherapy is also being studied in CRC. Checkpoint inhibitors of CTLA4 and PD1/PDL1 were studied in a phase I trial, but the results are not so encouraging in CRC patients. In a phase I study that included 296 advanced solid cancer patients out of which 19patients are colorectal. No objective response is noted in the CRC patients (84). Later a phase II study was done to evaluate the clinical activity of pembrolizumab (anti-PD1) in 41 patients with progressive metastatic carcinoma with or without mismatch-repair deficiency. For mismatch repair deficient patients objective response rate and progression-free survival rate were 40% (4 of 10 patients) and 78% (7 of 9 patients) respectively, and mismatch repair-proficient CRCs was 0% (0 of 18 patients) and 11% (2 of 18 patients) respectively (hazard ratio for disease progression or death, 0.10 with P<0.001). Whole genome exon sequencing showed high somatic mutational load of 1,782 vs. 73 in mismatch repair deficient to mismatch repair proficient tumors. High somatic mutation load was associated with increased PFS (85).
Trials including combinations with biological agents, vaccines and chemotherapy are under progress. The future may hold promising results for immunotherapy in CRC. Table 1 illustrates the ongoing randomized phase II/III trials of angiogenesis inhibitors in advanced CRC.
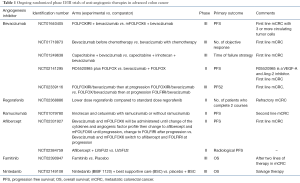
Full table
Conclusions
Anti-angiogenic therapy plays a significant role in the treatment of mCRC. The currently available anti-angiogenic treatments are bevacizumab, ziv-aflibercept, regorafenib and ramucirumab. Bevacizumab can be used in different settings including first line, second line, during maintenance and beyond PD. Ziv-aflibercept can be used along with irinotecan-based regimens in the second line setting. Regorafenib is used as a salvage therapy in mCRC. The most recent approval is ramucirumab for use in combination with FOLFIRI in patients whose disease has progressed during or after therapy with bevacizumab, oxaliplatin and fluoropyrimidine. Many novel anti-angiogenic agents are in various phases of development. Though we have achieved progress, the median OS for advanced CRC is 2.5 years. The effect of anti-angiogenic therapy is short-lived and patients eventually progress. Various resistance mechanisms are being studied and this will guide future research to overcome the problem. Also the challenges that remain are the lack of standardized biomarkers predicting the response to anti angiogenesis. The development of predictive biomarkers, molecular insights in emerging novel and combination therapies may help improve cure rates in advanced CRC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015 CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002;29:15-8. [Crossref] [PubMed]
- Konda B, Shum H, Rajdev L. Anti-angiogenic agents in metastatic colorectal cancer. World J Gastrointest Oncol 2015;7:71-86. [Crossref] [PubMed]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:1182-6. [Crossref] [PubMed]
- Verheul HM, Pinedo HM. The role of vascular endothelial growth factor (VEGF) in tumor angiogenesis and early clinical development of VEGF-receptor kinase inhibitors. Clin Breast Cancer 2000;1:S80-4. [Crossref] [PubMed]
- Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983;219:983-5. [Crossref] [PubMed]
- Leung DW, Cachianes G, Kuang WJ, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989;246:1306-9. [Crossref] [PubMed]
- Shinkaruk S, Bayle M, Laïn G, et al. Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy. Curr Med Chem Anticancer Agents 2003;3:95-117. [Crossref] [PubMed]
- Jayson GC, Kerbel R, Ellis LM, et al. Antiangiogenic therapy in oncology: current status and future directions. Lancet 2016;388:518-29. [Crossref] [PubMed]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev 1997;18:4-25. [Crossref] [PubMed]
- Dimova I, Popivanov G, Djonov V. Angiogenesis in cancer - general pathways and their therapeutic implications. J BUON 2014;19:15-21. [PubMed]
- Hicklin DJ, Ellis LM. Role of the Vascular Endothelial Growth Factor Pathway in Tumor Growth and Angiogenesis. J Clin Oncol 2005;23:1011-27. [Crossref] [PubMed]
- Kerbel RS. Tumor angiogenesis N Engl J Med 2008;358:2039-49. [Crossref] [PubMed]
- Escudier B, Szczylik C, Porta C, et al. Treatment selection in metastatic renal cell carcinoma: expert consensus Nat Rev Clin Oncol 2012;9:327-37. [Crossref] [PubMed]
- Dvorak HF, Brown LF, Detmar M, et al. Vascular permeability factor/vascular endothelial growth factor, micro vascular hyper permeability, and angiogenesis. Am J Pathol 1995;146:1029-39. [PubMed]
- Mancuso A, Sternberg CN. Colorectal cancer and antiangiogenic therapy: what can be expected in clinical practice? Crit Rev Oncol Hematol 2005;55:67-81. [Crossref] [PubMed]
- Reinmuth N, Parikh AA, Ahmad SA, et al. Biology of Angiogenesis in tumors of the GI tract. Microsc Res Tech 2003;60:199-207. [Crossref] [PubMed]
- Dienstmann R, Salazar R, Tabernero J. Personalizing colon cancer adjuvant therapy: selecting optimal treatments for individual patients. J Clin Oncol 2015;33:1787-96. [Crossref] [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [Crossref] [PubMed]
- Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 2003;21:60-5. [Crossref] [PubMed]
- Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol 2005;23:3697-705. [Crossref] [PubMed]
- McCormack PL, Keam SJ. Bevacizumab: a review of its use in metastatic colorectal cancer. Drugs 2008;68:487-506. [Crossref] [PubMed]
- Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-9. [Crossref] [PubMed]
- Petrelli F, Borgonovo K, Cabiddu M, et al. FOLFIRI-bevacizumab as first-line chemotherapy in 3500 patients with advanced colorectal cancer: a pooled analysis of 29 published trials. Clin Colorectal Cancer 2013;12:145-51. [Crossref] [PubMed]
- Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014;371:1609-18. [Crossref] [PubMed]
- Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 2015;16:1306-15. [Crossref] [PubMed]
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25:1539-44. [Crossref] [PubMed]
- Díaz-Rubio E, Gómez-España A, Massutí B, et al. First-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single-agent bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: the phase III MACRO TTD study. Oncologist 2012;17:15-25. [Crossref] [PubMed]
- Simkens LH, van Tinteren H, May A, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomized controlled trial of the Dutch Colorectal Cancer Group. Lancet 2015;385:1843-52. [Crossref] [PubMed]
- Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol 2008;26:5326-34. [Crossref] [PubMed]
- Grothey A, Flick ED, Cohn AL, et al. Bevacizumab exposure beyond first disease progression in patients with metastatic colorectal cancer: analyses of the ARIES observational cohort study. Pharmacoepidemiol Drug Saf 2014;23:726-34. [Crossref] [PubMed]
- Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomized phase 3 trial. Lancet Oncol 2013;14:29-37. [Crossref] [PubMed]
- Masi G, Salvatore L, Boni L, et al. Continuation or reintroduction of bevacizumab beyond progression to first-line therapy in metastatic colorectal cancer: final results of the randomized BEBYP trial. Ann Oncol 2015;26:724-30. [Crossref] [PubMed]
- Otten HM, Prins MH, Smorenburg SM, et al. Risk assessment and prophylaxis of venous thromboembolism in non-surgical patients: cancer as a risk factor. Haemostasis 2000;30 Suppl 2:72-6; discussion 63. [PubMed]
- Leighl NB, Bennouna J, Yi J, et al. Bleeding events in bevacizumab-treated cancer patients who received full-dose anticoagulation and remained on study. Br J Cancer 2011;104:413-8. [Crossref] [PubMed]
- Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA 2002;99:11393-8. [Crossref] [PubMed]
- Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012;15:171-85. [Crossref] [PubMed]
- Perkins SL, Cole SW. Ziv-aflibercept (Zaltrap) for the Treatment of Metastatic Colorectal cancer. Ann Pharmacother 2014;48:93-8. [Crossref] [PubMed]
- Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30:3499-506. [Crossref] [PubMed]
- Tang PA, Cohen SJ, Kollmannsberger C, et al. Phase II clinical and pharmacokinetic study of aflibercept in patients with previously treated metastatic colorectal cancer. Clin Cancer Res 2012;18:6023-31. [Crossref] [PubMed]
- Pericay C, Folprecht G, Saunders M, et al. Phase 2 randomized, noncomparative, open-label study of aflibercept and modified Folfox6 in the first-line treatment of metastatic colorectal cancer (AFFIRM) Ann Oncol 2012;23:iv5-iv18. [PubMed]
- Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245-55. [Crossref] [PubMed]
- Mross K, Frost A, Steinbild S, et al. A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res 2012;18:2658-67. [Crossref] [PubMed]
- Strumberg D, Scheulen ME, Schultheis B, et al. Regorafenib (BAY 73-4506) in advanced colorectal cancer: a phase I study. Br J Cancer 2012;106:1722-7. [Crossref] [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [Crossref] [PubMed]
- Zaniboni A. New active drugs for the treatment of advanced colorectal cancer. World J Gastrointest Surg 2015;7:356-9. [Crossref] [PubMed]
- Tabernero J, Yoshino T, Cohn AL, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol 2015;16:499-508. [Crossref] [PubMed]
- Tampellini M, Sonetto C, Scagliotti GV. Novel anti-angiogenic therapeutic strategies in colorectal cancer. Expert Opin Investig Drugs 2016;25:507-20. [Crossref] [PubMed]
- Xu RH, Shen L, Wang K, et al. A randomized, double-blind, parallel-group, placebo-controlled, multicenter, phase II clinical study of famitinib in the treatment of advanced metastatic colorectal cancer. J Clin Oncol 2015;33:abstr 513.
- Reck M. Nintedanib: examining the development and mechanism of action of a novel triple angiokinase inhibitor. Expert Rev Anticancer Ther 2015;15:579-94. [Crossref] [PubMed]
- Van Cutsem E, Prenen H, D'Haens G, et al. A phase I/II, open-label, randomised study of nintedanib plus mFOLFOX6 versus bevacizumab plus mFOLFOX6 in first-line metastatic colorectal cancer patients. Ann Oncol 2015;26:2085-91. [Crossref] [PubMed]
- Van Cutsem E, Yoshino T, Hocke J, et al. Rationale and Design for the LUME-Colon 1 Study: A Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Nintedanib Plus Best Supportive Care Versus Placebo Plus Best Supportive Care in Patients With Advanced Colorectal Cancer Refractory to Standard Treatment. Clin Colorectal Cancer 2016;15:91-94.e1. [Crossref] [PubMed]
- Samalin E, Bouché O, Thézenas S, et al. Sorafenib and irinotecan (NEXIRI) as second- or later-line treatment for patients with metastatic colorectal cancer and KRAS-mutated tumors: a multicentre Phase I/II trial. Br J Cancer 2014;110:1148-54. [Crossref] [PubMed]
- Tabernero J, Garcia-Carbonero R, Cassidy J, et al. Sorafenib in combination with oxaliplatin, leucovorin, and fluorouracil (modified FOLFOX6) as first-line treatment of metastatic colorectal cancer: the RESPECT trial. Clin Cancer Res 2013;19:2541-50. [Crossref] [PubMed]
- Heng DY, Kollmannsberger C. Sunitinib. Recent Results Cancer Res 2010;184:71-82. [Crossref] [PubMed]
- Van Cutsem E, Bajetta E, Valle J, et al. Randomized, placebo-controlled, phase III study of oxaliplatin, fluorouracil, and leucovorin with or without PTK787/ZK 222584 in patients with previously treated metastatic colorectal adenocarcinoma. J Clin Oncol 2011;29:2004-10. [Crossref] [PubMed]
- Hecht JR, Trarbach T, Hainsworth JD, et al. Randomized, placebo-controlled, phase III study of first-line oxaliplatin-based chemotherapy plus PTK787/ZK 222584, an oral vascular endothelial growth factor receptor inhibitor, in patients with metastatic colorectal adenocarcinoma. J Clin Oncol 2011;29:1997-2003. [Crossref] [PubMed]
- Liu S, Kurzrock R. Toxicity of targeted therapy: implications for response and impact of genetic polymorphisms. Cancer Treat Rev 2014;40:883-91. [Crossref] [PubMed]
- Saif MW, Mehra R. Incidence and management of bevacizumab-related toxicities in colorectal cancer. Expert Opin Drug Saf 2006;5:553-66. [Crossref] [PubMed]
- Bécouarn Y, Cany L, Pulido M, et al. FOLFIRI® and Bevacizumab in first-line treatment for colorectal cancer patients: safety, efficacy and genetic polymorphisms. BMC Research Notes 2014;7:260. [Crossref] [PubMed]
- Carter NJ. Regorafenib: a review of its use in previously treated patients with progressive metastatic colorectal cancer. Drugs Aging 2014;31:67-78. [Crossref] [PubMed]
- Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy Clin Cancer Res 2008;14:6371-5. [Crossref] [PubMed]
- Ribatti D. Tumor refractoriness to anti-VEGF therapy. Oncotarget 2016. [Epub ahead of print]. [PubMed]
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005;307:58-62. [Crossref] [PubMed]
- Akiyama K, Ohga N, Hida Y, et al. Tumor endothelial cells acquire drug resistance by MDR1 up-regulation via VEGF signaling in tumor microenvironment Am. J. Pathol 2012;180:1283-93. [Crossref] [PubMed]
- Huang L, Hu C, Di Benedetto M, et al. Induction of multiple drug resistance in HMEC-1 endothelial cells after long-term exposure to sunitinib. Onco Targets Ther 2014;7:2249-55. [PubMed]
- Croci DO, Cerliani JP, Dalotto-Moreno T. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell 2014;156:744-58. [Crossref] [PubMed]
- Huijbers EJ, van Beijnum JR, Thijssen VL, et al. Role of the tumor stroma in resistance to anti-angiogenic therapy. Drug Resist Updat 2016;25:26-37. [Crossref] [PubMed]
- Dey N, De P, Brian LJ. Evading anti-angiogenic therapy: resistance to anti-angiogenic therapy in solid tumors. Am J Transl Res 2015;7:1675-98. [PubMed]
- Casanovas O, Hicklin DJ, Bergers G, et al. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 2005;8:299-309. [Crossref] [PubMed]
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 2008;8:592-603. [Crossref] [PubMed]
- Jubb AM, Turley H, Moeller HC, et al. Expression of delta-like ligand 4 (Dll4) and markers of hypoxia in colon cancer. Br J Cancer 2009;101:1749-57. [Crossref] [PubMed]
- Noguera-Troise I, Daly C, Papadopoulos NJ, et al. Blockade of DLL4 inhibits tumor growth by promoting non-productive angiogenesis. Nature 2006;444:1032-7. [Crossref] [PubMed]
- Ridgway J, Zhang G, Wu Y, et al. Inhibition of DLL4 signalling inhibits tumor growth by deregulating angiogenesis. Nature 2006;444:1083-7. [Crossref] [PubMed]
- Cidon EU, Alonso P, Masters B. Markers of response to antiangiogenic therapies in colorectal cancer: where are we now and what should be next? Clin Med Insights Oncol 2016;10:41-55. [Crossref] [PubMed]
- Österlund P, Soveri LM, Isoniemi H, et al. Hypertension and overall survival in metastatic colorectal cancer patients treated with bevacizumab-containing chemotherapy. Br J Cancer 2011;104:599-604. [Crossref] [PubMed]
- Tahover E, Uziely B, Salah A, et al. Hypertension as a predictive biomarker in bevacizumab treatment for colorectal cancer patients Med Oncol 2013;30:327. [Crossref] [PubMed]
- Scartozzi M, Galizia E, Chiorrini S, et al. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol 2009;20:227-30. [Crossref] [PubMed]
- Jubb AM, Hurwitz HI, Bai W, et al. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol 2006;24:217-27. [Crossref] [PubMed]
- Wang Y, Yao X, Ge J, et al. Can vascular endothelial growth factor and microvessel density be used as prognostic biomarkers for colorectal cancer? A systematic review and meta-analysis. ScientificWorldJournal 2014;2014:102736.
- Drevs J, Schneider V. The use of vascular biomarkers and imaging studies in the early clinical development of anti-tumor agents targeting angiogenesis. J Intern Med 2006;260:517-29. [Crossref] [PubMed]
- Jain RK, Duda DG, Willett CG, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol 2009;6:327-38. [Crossref] [PubMed]
- Suenaga M, Mashima T, Kawata N, et al. Serum VEGF-A and CCL5 levels as candidate biomarkers for efficacy and toxicity of regorafenib in patients with metastatic colorectal cancer. Oncotarget 2016. [Epub ahead of print]. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
Cite this article as: Nandikolla AG, Rajdev L. Targeting angiogenesis in gastrointestinal tumors: current challenges. Transl Gastroenterol Hepatol 2016;1:67.


