A rare case of a primary hepatic neuroendocrine tumor
Background
Neuroendocrine tumors are well differentiated low grade malignant neoplasms. Their pathogenesis is thought to be secondary to the unrestricted proliferation of neuroendocrine cells (1). They most commonly arise in the bronchopulmonary or gastrointestinal tract but can originate from almost any organ (2). While the liver is a common site of metastasis, primary hepatic neuroendocrine tumors are an exceedingly rare pathology, of which fewer than 100 cases have been described in literature since they were first reported by Lubarsch et al. in 1888 (3). Thus, there exists a paucity of data regarding the clinical presentation, diagnosis and management of this disease. We present a patient with a non-secretory primary hepatic neuroendocrine tumor who was successfully managed with surgical resection and remains disease free over 36 months after initial presentation.
Case presentation
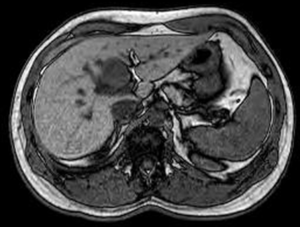
A 35-year-old health care worker presented to our facility for evaluation of a cough. On exam cervical lymphadenopathy was noted. The patient also reported the development of a non-pruritic maculopapular rash along the anterior, posterior chest and bilateral upper extremities. The patient did not demonstrate any clinical symptoms and denied any nausea, vomiting, fevers, chills, weight loss, night sweats, flushing or abdominal pain. Secondary to concern for an underlying malignancy, patient underwent hematology and oncology workup including a computer tomography (CT) of the abdomen, somatostatin-receptor scintigraphy and core biopsy. Biopsy of the lymph node resulted in non-specific reactive findings, however, on abdominal CT a hypodense heterogeneous hypervascular lesion measuring 3.7 cm × 2.7 cm in segment IVb of the liver was noted (Figure 1). Somatostatin receptor scintigraphy noted no areas of radioisotope uptake. No vascular involvement was seen. Mild intrahepatic biliary dilation was noted proximal to the lesion. This was confirmed with MRI. Initial differential diagnosis included hepatocellular carcinoma, cholangiocarcinoma, hepatic adenoma, metastasis, angiosarcoma, hemangiopericytoma, and a neuroendocrine tumor. The levels of laboratory studies such as liver function tests, alkaline phosphatase, chromogranin A (4.0 ng/mL), 24-hour 5 hydroxyindoleacetic acid (5-HIAA) (1.8 mg) and tumor markers including alpha-fetoprotein (2.23 ng/mL), were not elevated. HIV test was negative. Follow-up MRI scan was intermediate and the patient underwent CT guided biopsy of the hepatic lesion. Immunohistochemical staining from the biopsy resulted in cells reactive for synaptophysin, chromogranin, anti-Cytokeratin (CAM 5.2), MOC31, CD 56 and mucin glycoprotein 1 (MUC 1) and non-reactive for CD 34, CD31 and CD 30. Hematoxylin and eosin staining narrowed the differential diagnosis to vascular verses neuroendocrine tumors. The immunohistochemical profile confirmed a diagnosis of neuroendocrine tumor (Figures 2,3). We sent the biopsy to our affiliated cancer center where initial pathology was confirmed. Thereafter, patient underwent PET scan, CT chest, MRI head along with EUS, EGD and colonoscopy to evaluate for a primary source and all modalities were negative. As a result of the radiological and immunohistochemical evaluation, a diagnosis of primary hepatic non secreting neuroendocrine tumor was established. Neck mass spontaneously resolved.
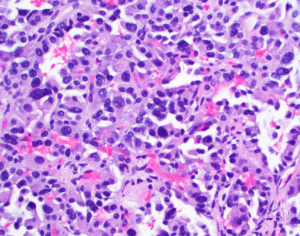
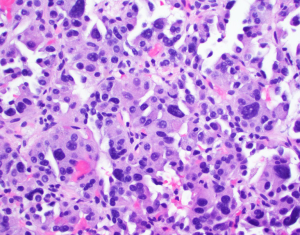
The well localized presentation without extensive hepatic invasion made the patient a candidate for surgical resection. Resection of the tumor was performed with Roux-en-Y hepaticojejunostomy. No additional adjuvant therapy was recommended. Surgical margins were negative. Post-surgical follow-up was complicated with three enteric biliary anastomosis requiring dilation and drain placement. Patient received post-operative follow-up and recurrence screening. No other symptoms or imaging findings were noted as of 36 months from presentation.
Discussion
Hepatic neuroendocrine tumors are a rare pathology whose diagnosis is often missed on initial evaluation. Their exact origin is unknown with literature suggesting origination from ectopic pancreatic or adrenal tissue in the liver or intestinal metaplasia of intrahepatic neuroendocrine cells (1). Neuroendocrine tumors most commonly originate in the gastrointestinal system, mostly in the appendix or small bowel (2,4). Less commonly they may be seen in the bronchopulmonary system, pancreas and genitals as well. The rarity of primary hepatic neuroendocrine tumors leads to a low index of suspicion on initial evaluation making diagnosis prior to biopsy or resection difficult. This was the first case identified at our 1,000 bed tertiary care center. An accurate diagnosis is essential relating to the small but significant portion of malignant tumors. Classification of these tumors is based on histopathological features. Type I includes well-differentiated tumors of low grade malignancy with an indolent development and a good prognosis. Type II includes moderately differentiated or intermediate grade neoplasms, and poorly differentiated or high grade epithelial neoplasms that carry a poor prognosis as classified as type III (4).
The most common symptom on presentation is abdominal pain, however, the second most common presentation is with no symptoms at all (1). Additionally, patients may present with palpable mass, weight loss, Cushing syndrome and carcinoid syndrome. Concomitant underlying liver pathology such HBV or HCV infection has been noted in the past (5). They are most commonly an incidental finding, are more prevalent in females and patients over the age of 50 (age range, 8–83 years) (1,4). These tumors are characterized by a slow growth pattern and are most commonly nonfunctional. When functional, they have the ability to secrete hormones with gastrin (10.1%) followed by chromogranin A being the most common (6).
On radiologic exam, primary hepatic neuroendocrine tumors present as solitary lesions >3 cm with a multi-nodular appearance more suggestive of metastasis. The right lobe is the most common location however, left lobe and bilateral lobe tumors may also be seen more rarely (6). Due to their histological similarity, a misdiagnosis as hepatocellular carcinoma is common, illustrating the need for immunohistochemical staining (5).
Surgical resection is the preferred treatment modality in these patients (4). The inclusion criteria is the same as that developed for liver tumors (1). Favorable long-term outcomes have been described with this treatment (7,8). Liver transplant may be required in a small number of cases if not amenable to surgical resection especially if associated with hormone secretion, vascular involvement or unresponsive to more conservative options (6,9). Post liver transplant, patients remained asymptomatic for 38–95 months (1,7). For patients with unresectable disease, a non-surgical approach may be preferred. This involves the use of ablation, hepatic artery embolization and somatostatin analogues (10,11). Octreotide, a somatostatin analog may not only alleviate hormonal symptoms but may also have an anti-proliferative effect (12). Resection of the tumor has been shown to have good 10 year survival of 68% as compared to 59% for all patients. Furthermore, the administration of preoperative chemotherapy, radiation or chemoembolization is not impact patient survival (13). Other factors such as age, gender, presence of extrahepatic metastasis, number of tumors, or distribution of the tumor within the liver have not been shown to impact long-term patient survival. Even patients with positive tumor margins on biopsy have been shown benefit from surgical resection (14).
Conclusions
Primary hepatic neuroendocrine tumors are a rare entity, which if appropriately diagnosed and treated results in positive patient outcomes. Due to their uncommon presentation, it is important to exclude other possible etiologies prior to making a definitive diagnosis. Once identified, excluding other primary locations with thorough investigation and treatment with surgical resection has been shown to provide the most patient benefit.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Gravante G, De Liguori Carino N, et al. Primary carcinoids of the liver: a review of symptoms, diagnosis and treatments. Dig Surg 2008;25:364-8. [Crossref] [PubMed]
- Scarsbrook AF, Ganeshan A, Statham J, et al. Anatomic and functional imaging of metastatic carcinoid tumors. Radiographics 2007;27:455-77. [Crossref] [PubMed]
- Lubarsch O. Uberdenprimäeren Krebs desileum, nebstbemerkungenüber das gleichzeitigevorkommenvonkrebsund tuberculose. Virchows Arch 1888;111:280-370. [Crossref]
- Landen S, Elens M, Vrancken C, et al. Giant hepatic carcinoid: a rare tumor with a favorable prognosis. Case Rep Surg 2014;2014:456509.
- Lin CW, Lai CH, Hsu CC, et al. Primary hepatic carcinoid tumor: a case report and review of the literature. Cases J 2009;2:90. [Crossref] [PubMed]
- Lehnert T. Liver transplantation for metastatic neuroendocrine carcinoma: an analysis of 103 patients. Transplantation 1998;66:1307-12. [Crossref] [PubMed]
- Fenwick SW, Wyatt JI, Toogood GJ, et al. Hepatic resection and transplantation for primary carcinoid tumors of the liver. Ann Surg 2004;239:210-9. [Crossref] [PubMed]
- Norton JA, Warren RS, Kelly MG, et al. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery 2003;134:1057-63; discussion 1063-5. [Crossref] [PubMed]
- Le Treut YP, Delpero JR, Dousset B, et al. Results of liver transplantation in the treatment of metastatic neuroendocrine tumors. A 31-case French multicentric report. Ann Surg 1997;225:355-64. [Crossref] [PubMed]
- Touloumis Z, Delis SG, Triantopoulou C, et al. Primary hepatic carcinoid; a diagnostic dilemma: a case report. Cases J 2008;1:314. [Crossref] [PubMed]
- Krishnamurthy SC, Dutta V, Pai SA, et al. Primary carcinoid tumor of the liver: report of four resected cases including one with gastrin production. J Surg Oncol 1996;62:218-21. [Crossref] [PubMed]
- Wängberg B, Nilsson O, Johanson V V, et al. Somatostatin Receptors in the Diagnosis and Therapy of Neuroendocrine Tumor. Oncologist 1997;2:50-58. [PubMed]
- Knox CD, Anderson CD, Lamps LW, et al. Long-term survival after resection for primary hepatic carcinoid tumor. Ann Surg Oncol 2003;10:1171-5. [Crossref] [PubMed]
- Glazer ES, Tseng JF, Al-Refaie W, et al. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB (Oxford) 2010;12:427-33. [Crossref] [PubMed]
Cite this article as: Sethi S, Kulkarni P. A rare case of a primary hepatic neuroendocrine tumor. Transl Gastroenterol Hepatol 2016;1:66.