Mucinous adenocarcinoma of perianal region: an uncommon disease treated with neo-adjuvant chemo-radiation
Introduction
Mucinous adenocarcinoma of the perianal region is an extremely rare occurrence accounting for 2% to 3% of all gastrointestinal malignancies (1,2). With no uniform consensus till date regarding the origin, pathogenesis and biological behavior of this pathology, it remains a diagnostic and therapeutic challenge for most of us. They are known to be associated with benign inflammatory conditions like chronic anal fistulae, perianal abscesses and Crohn’s disease (3) which makes it difficult for an early diagnosis and subsequent management as patients present with a highly advanced stage. These are locally aggressive neoplasm with a poor prognosis as the exact management protocol is still unknown due to lack of any clinical controlled trials. It is said to have a slow indolent course with low incidence lymph node spread (4) or distant metastasis (5). However Gaertner et al. (2) in 14 cases of anal adenocarcinoma described 6 patients with lymph nodes metastases and 2 developing distant metastasis. Patients generally present with bleeding during defecation, perianal pain and necrotic perianal ulcerative lesion in cases of progressive disease. Management of patients suffering from this entity generally has been surgical in the form of abdominoperineal resection (APR) in an upfront setting (6,7). However the role of neoadjuvant and adjuvant therapy remains undefined in this disease (7). Since in our case complete resection of the lesion was difficult due to its large size and surrounding tissue infiltration, we used neoadjuvant concurrent chemo-radiotherapy (NACCRT) followed by surgery and adjuvant chemotherapy on the lines of locally advanced rectal adenocarcinomas. The aim of reporting this case is to highlight its rarity, clinicopathological characteristics and the treatment provided with its final outcome and subsequent follow-up.
Case representation
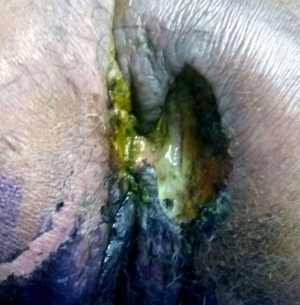
A 56-year-old male presented with history of painful defecation associated with mucoid discharge and occasional bleeding per rectum with a growth in the perianal region of 4 months duration with no history of any altered bowel habits. He gave a past history of recurrent fistula in ano over 2 years for which he had taken alternate medications and later underwent local incision and drainage for the same. However over time he developed mucus discharging perianal mass at the surgical site. His hematological and biochemical parameters were within normal range. Clinical evaluation showed a large ulcerative growth measuring 8 cm × 6 cm with surrounding induration and external fistula openings in the perianal region and another lesion measuring 3 cm × 2 cm at 7 o’clock position (Figure 1). Digital rectal examination showed no growth in anorectum with a lax anal opening and no inguinal or any generalized lymphadenopathy. Serum carcinoembryonic antigen (CEA) level was raised with a value of 71.74 ng/mL.
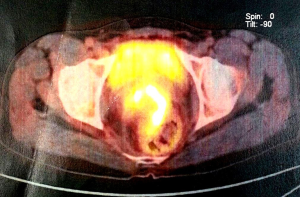
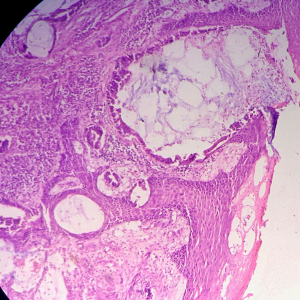
Biopsy from the lesion revealed well differentiated dilated tortuous glands with lakes of mucin and intervening stroma showing moderate lymphoplasmacytic infiltration and desmoplastic reaction consistent with mucinous adenocarcinoma (Figure 2). Computed tomography (CT) scan showed a large ulcer in the perianal region with a collection in the right ischiorectal fossa (Figures 3,4). No growth was seen in anal canal or rectum without any pelvic or inguinal lymphadenopathy. Metastatic workup with a positron emission tomography (PET) scan was negative and showed a localized lesion in the perianal region (Figure 5). Colonoscopy showed no obvious malignant growth in the colon, rectum and anal canal (Figure 6) thus confirming the diagnosis of perianal malignancy. The clinical stage was cT4N0M0.
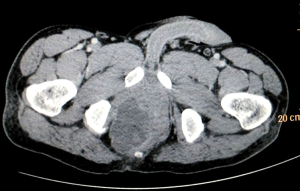
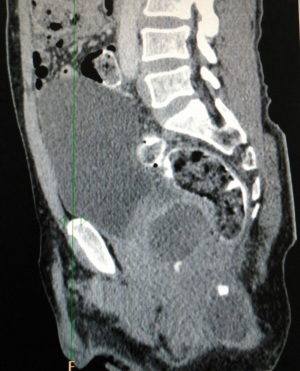

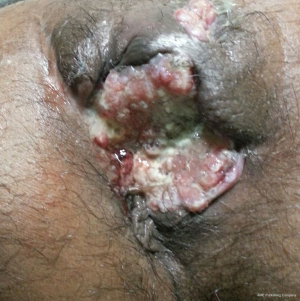
After confirmation of diagnosis, management options were discussed and in view of extensive involvement of surrounding soft tissues and anal canal, upfront surgery was not considered due to the possibility of a R2 resection. With the pathological diagnosis of adenocarcinoma, patient was treated with upfront radiation therapy (RT) by 3-dimensional conformal radiotherapy (3-DCRT) to a dose of 50.4 Gray (Gy) in 28 fractions with concurrent chemotherapy capecitabine in a neoadjuvant setting. Patient received oral capecitabine to a dose of 825 mg/m2 twice daily 5 days/week with RT for 5 weeks. A reassessment of the patient 6 weeks post stipulated NACCRT showed significant response clinically (Figure 7) and a partial response (PR) on imaging which showed more than 30% decrease in the longest diameter of the target lesion as per revised RECIST guidelines version 1.1. He underwent surgery after 8 weeks in form of APR with excision of the skin surrounding the lesion. The length of the proximal margin was 5 cm while the distal margin was negative for any tumor deposits. The resected specimen showed mucinous adenocarcinoma extending to ischiorectal fossa and anal sphincter with no evidence of rectal, anal canal or lymph nodal involvement with a pathological staging of ypT3N0M0. The patient was then exhibited adjuvant oral capecitabine at 1,250 mg/m2 twice daily, day 1–14 every 3 weeks for 6 months. Presently kept on 3 monthly follow-up since last 1 year post APR and adjuvant chemotherapy with no evidence of local recurrence or distant metastasis.
Discussion
Perianal mucinous adenocarcinoma is a very uncommon entity accounting for only 2% to 3% of all gastrointestinal tumors (1,2) and 3% to 11% of perianal cancers (4). This entity poses a diagnostic and therapeutic dilemma as till date no clear consensus has been reached regarding its etiopathogenesis and disease classification thereby hindering subsequent management. They are known to be associated with benign inflammatory conditions like chronic anal fistulae, perianal abscesses, syphilis, diabetes, tuberculosis and lymphogranuloma venereum with an incidence of 0.7% in patients with perineal Crohn’s disease (3). The association of perianal carcinoma with a long standing anal fistula was first described in 1934 by Rosser (8) in 7 patients. Skir (9) in 1948 described 2 patients, Heidenreich et al. (10) in 1966 reported 2 such cases while Gaertner et al. (2) in 2008 reported 14 cases of anal adenocarcinoma associated with chronic fistula. The clinical presentation of this disease may be perianal pain or itching, painful defecation, stool mixed with blood or mucus, bleeding per rectum, obstruction due to the growth obscuring the anal opening or an ulcero-proliferative growth or palpable mass in the perianal region with bloody or mucoid discharge. The most frequent clinical presentation is the symptoms related with a longstanding fistula of more than 10 years (8). Our case is very unique in the sense that the patient presented with a history of preceding anal fistula of only 2 years duration in contrast to previous case reports and series which have established the preset duration of more than 10 years.
Regarding its pathogenesis, the association of malignancy with chronic anal fistulas may be either due a tumor located in anorectum or colon, a cancer may itself present as a fistula or a cancer may proliferate in an anal fistula. Rosser (8) postulated three criteria for describing the pathophysiology of this unusual entity; the fistula may precede the carcinoma by at least 10 years, the fistula opens inside the anal canal and outside the tumor and any anorectal cancer should be an extension from the fistula harboring the carcinoma. Few cases have reported anal ducts and glands as the origin of perianal pathology (11). These ducts and glands may pierce the internal sphincter muscle before they extend to the perianal area in the ischiorectal fossa thus forming anal fistulas and perianal abscesses. Due to repeated friction, scarring and inflammatory reactions, the glands or ducts transform into mucinous carcinomas though there is no definite data to support this theory. But these can be predisposing factors for development of fistula in ano. Dukes and Galvin (12) in 1956 gave the theory that this cancer originated from intestinal rests of cells though the natural history of the disease is not well known. No relation has been seen with risk factors associated with colorectal adenocarcinomas like smoking, alcohol consumption, metabolic syndromes, obesity and low levels of physical activity or dietary intake of red meat. Also no age or sex predilection has been documented so far.
The early diagnosis of perianal adenocarcinoma is difficult as the tumor has an indolent growth and remains hidden within the perianal region. However it can be suspected with the formation of new ulcero-proliferative growth in a case of persistent fistula in ano. Apart from the routine hematological and biochemical parameters, a complete evaluation of the entire gastro-intestinal tract is essential with no growth in anorectum on proctoscopic or colonoscopic examination to assume the diagnosis. Imaging modalities like endoscopic ultrasound (EUS), CT scan and magnetic resonance imaging (MRI) help determining the disease extension to adjacent tissues while functional imaging technique like PET scan aids in metastatic mapping of the disease, though metastatic dissemination is rare (5). For mucinous tumors PET is not the first line evaluation technique as compared to gold standard MRI or CT scan because the presence of mucin causes considerable number of false negative results. The 2007 National Comprehensive Cancer Network treatment guidelines included PET scan as part of the standard pre-treatment work of patients diagnosed with anal carcinoma. The new version 2.2012 considers PET for workup but its use for treatment planning or staging has not been validated. In a series by Mistrangelo M (13), tumors were staged according to AJCC. PET-CT up-staged 9–100% and down-staged 0–25% of patients studied. The radiation fields changed in 3.7–33% of cases. We did a PET scan as the carcinoembryonic antigen (CEA) level was raised. The CEA levels are part of perioperative workup as they can be raised indicating a local disease spread or serve as a marker of metastasis (14). The definite diagnosis of this disease entity is primarily by histopathological evaluation of the biopsied tissue which should be obtained from the perianal lesion instead of the fistulous tract as it could be misleading. The presence of well differentiated dilated tortuous glands filled with lakes of mucin infiltrating dermal or muscular elements confirms the diagnosis.
Management options for perianal adenocarcinomas have been described by Ilbawi et al. (14) to be associated with its subtypes. Anal duct pathology, the known aggressive variety has been managed with APR followed by adjuvant concurrent chemo-radiation (CCRT) or by NACCRT followed by APR. Like-wise perianal adenocarcinoma associated with ectopic or remnant anal glands can have high local recurrence and warrants APR with wide local excision (WLE) of perianal tissue to achieve negative margins. Extramucosal perianal adenocarcinoma developing in chronic fistula was treated with WLE only. Nielsen et al. (15) declared APR to be the definite therapeutic option regardless of the tumor subtype in view of involvement of anal sphincter, high rates of local recurrence and achieving a negative surgical margin. Inguinal lymph nodes are commonly involved as compared to pelvic lymph nodes in perianal adenocarcinoma (14). However, the overall incidence of lymph node involvement is low as compared to anorectal carcinomas (4) although Gaertner et al. (2) in 14 cases of anal adenocarcinoma described 6 patients with lymph nodal metastases. Presence of advanced disease along with lymphadenopathy confers a poor prognosis as the probability of sphincter preservation (16) and achieving a negative margin becomes bleak (14). Surgery is generally the preferred therapeutic option as it helps in removing the tumor bulk thus reducing the chances of local recurrence. A WLE is sometimes considered in old patients (16), in cases where the anal sphincter is not involved and the disease is limited to the perianal area (14). However, APR is generally the standard procedure even in elderly and when the tumor has poor prognostic factors like lymphadenopathy (17). Sometimes an APR is combined with WLE to achieve optimal local control (14). Extra-levator abdominoperineal excision (ELAPE) is another surgical technique which was introduced to decrease the rates of positive margins in distal or low ano-rectal carcinomas. It involves reconstruction of the pelvic floor and is used mainly in extensive disease. Compared to APR, it showed more incidence of circumferential resection margin (CRM) positivity with no improvements in short-term oncological results (18). In view of excellent response post NACCRT, our patient was considered for APR instead of ELAPE.
Though surgery has maintained its role as the definite management modality, the exact role of RT and chemotherapy in perianal adenocarcinoma either in neoadjuvant or adjuvant setting is still not defined. Yang et al. (19) exhibited CCRT to 2 out of 3 patients of perianal mucinous adenocarcinoma who refused surgery. They reported that although radical surgery with APR remains the treatment of choice, combined therapy with chemo-radiation showed promising results. In a retrospective review by Belkacémi et al. (20) of 82 patients of primary adenocarcinoma of the anal canal, they concluded that the disease requires rigorous management. Multivariate analysis showed that T and N stage, histologic grade and treatment modality are independent prognostic factors for survival. They observed better survival rates after combined chemo-radiation and recommended APR as a salvage approach. Most case reports about this disease describe a highly advanced lesion with surrounding tissue invasion and obtaining a negative margin or R0 resection becomes difficult as it happened in our case also. Presently NACCRT followed by surgery with APR and subsequent adjuvant chemotherapy has emerged as the mainstay of treatment for stage II and III rectal adenocarcinomas. NACCRT causes downsizing of large and advanced tumors, eradicates tumor cells that may be implanted into the surgical region or gets disseminated to systemic circulation, thus increases the chances of R0 resection and decreasing incidence of local recurrence (17). In view of tumor histology of adenocarcinoma and a large tumor invading surrounding soft tissue, upfront surgery was not considered and we followed the above therapeutic principle with significant response to NACCRT which was subsequently followed by APR in view of anal sphincter involvement and adjuvant chemotherapy.
Mucinous adenocarcinoma of the perianal region is an uncommon neoplasm of the gastrointestinal tract. By virtue of its anatomical location and histological variance, it oscillates between the pathogenesis of anal and rectal carcinomas causing a diagnostic and therapeutic uncertainty. In most of the cases as per the available literature CCRT is being used as an adjunct to surgery i.e., APR either in neoadjuvant or adjuvant setting. Though NACCRT has definitely improved the rates of R0 resection, CCRT being used as a definitive modality as in squamous cell carcinoma (SCC) of anal canal still needs to be explored. By reporting our case we want to suggest that newer screening modalities and prognostic markers should be developed to identify patients who are at risk for developing perianal carcinomas. Also a better understanding and interpretation of the molecular and biological mechanism of pathogenesis may help to device therapeutic strategies to counter this disease process. A high degree of clinical suspicion and histopathological confirmation is required to identify and diagnose any ulcero-proliferative growth in the perianal region with previous history of fistula in ano of any duration as a carcinoma so as to enable a prompt initiation of appropriate treatment. We also recommend NACCRT followed by surgery and adjuvant chemotherapy as an alternative to upfront surgery as the standard treatment guideline, though more prospective data favouring this recommendation is required.
Acknowledgements
The authors thank the patient for allowing them to publish the case report and use the images taken while he was treated in their institute. They also like to extend their gratitude to Department of Surgical Oncology, Department of Medical Oncology, Department of Nuclear Medicine, and Department of Radiology, Army Hospital Research and Referral, New Delhi, India.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Hongo K, Kazama S, Sunami E, et al. Perianal adenocarcinoma associated with anal fistula: a report of 11 cases in a single institution focusing on treatment and literature review. Hepatogastroenterology 2013;60:720-6. [PubMed]
- Gaertner WB, Hagerman GF, Finne CO, et al. Fistula-associated anal adenocarcinoma: good results with aggressive therapy. Dis Colon Rectum 2008;51:1061-7. [Crossref] [PubMed]
- Sjödahl RI, Myrelid P, Söderholm JD. Anal and rectal cancer in Crohn's disease. Colorectal Dis 2003;5:490-5. [Crossref] [PubMed]
- Okada K, Shatari T, Sasaki T, et al. Is histopathological evidence really essential for making a surgical decision about mucinous carcinoma arising in a perianal fistula? Report of a case. Surg Today 2008;38:555-8. [Crossref] [PubMed]
- Inoue Y, Kawamoto A, Okigami M, et al. Multimodality therapy in fistula-associated perianal mucinous adenocarcinoma. Am Surg 2013;79:e286-8. [PubMed]
- Leal RF, Ayrizono ML, Coy CS, et al. Mucinous adenocarcinoma derived from chronic perianal fistulas: report of a case and review of the literature. Tech Coloproctol 2007;11:155-7. [Crossref] [PubMed]
- Ibáñez J, Erro JM, Aranda F, et al. Mucinous adenocarcinoma on chronic perianal fistula treated by neoadjuvant chemoradiotherapy and laparoscopy-assisted abdominoperineal amputation. Cir Esp 2006;79:184-5. [PubMed]
- Rosser C. The relation of fistula in ano to cancer of the anal canal. Trans Am Proct Soc 1934;35:65-70, discussion 70-71.
- Skir I. Mucinous carcinoma associated with fistulas of long-standing. Am J Surg 1948;75:285-9. [Crossref] [PubMed]
- Heidenreich A, Collarini HA, Paladino AM, et al. Cancer in anal fistulas: report of two cases. Dis Colon Rectum 1966;9:371-6. [Crossref] [PubMed]
- Sierra EM, Villanueva Saenz E, Martínez PH, et al. Mucinous adenocarcinoma associated with fistula in ano: report of a case. Tech Coloproctol 2006;10:51-3. [Crossref] [PubMed]
- Dukes CE, Galvin C. Colloid carcinoma arising within fistulae in the anorectal region. Ann R Coll Surg Engl 1956;18:246-61. [PubMed]
- Mistrangelo M, Lesca A. PET-CT in Anal Cancer: Indications and Limits. In: Misciagna S, editor. Positron Emission Tomography - Recent Developments in Instrumentation, Research and Clinical Oncological Practice. InTech, 2013:235-56.
- Ilbawi AM, Simianu VV, Millie M, et al. Wide local excision of perianal mucinous adenocarcinoma. J Clin Oncol 2015;33:e16-8. [Crossref] [PubMed]
- Nielsen OV, Koch F. Carcinomas of the anorectal region of extramucosal origin with special reference to the anal ducts. Acta Chir Scand 1973;139:299-305. [PubMed]
- Yamaguchi T, Kagawa R, Sakata S, et al. Successful sphincter-sparing local excision for mucinous adenocarcinoma associated with chronic fistula in ano using preoperative MRI evaluation. Int Surg 2008;93:220-5. [PubMed]
- Santos MD, Nogueira C, Lopes C. Mucinous adenocarcinoma arising in chronic perianal fistula: good results with neoadjuvant chemoradiotherapy followed by surgery. Case Rep Surga 2014;2014:386150.
- Klein M, Fischer A, Rosenberg J, et al. Extralevatory abdominoperineal excision (ELAPE) does not result in reduced rate of tumor perforation or rate of positive circumferential resection margin: a nationwide database study. Ann Surg 2015;261:933-8. [Crossref] [PubMed]
- Yang BL, Shao WJ, Sun GD, et al. Perianal mucinous adenocarcinoma arising from chronic anorectal fistulae: a review from single institution. Int J Colorectal Dis 2009;24:1001-6. [Crossref] [PubMed]
- Belkacémi Y, Berger C, Poortmans P, et al. Management of primary anal canal adenocarcinoma: a large retrospective study from the Rare Cancer Network. Int J Radiat Oncol Biol Phys 2003;56:1274-83. [Crossref] [PubMed]
Cite this article as: Purkayastha A, Sharma N, Dutta V, Bisht N, Pandya T. Mucinous adenocarcinoma of perianal region: an uncommon disease treated with neo-adjuvant chemo-radiation. Transl Gastroenterol Hepatol 2016;1:52.