Laparoscopic pancreatic resection—a review
Introduction
In the last decades minimal access surgery (MAS) has gained wide spread use both for benign and malignant disease in gastrointestinal surgery (1). Oncological adequacy has been shown in a variety of indications, including colonic (2,3) and gastric cancer (4). Laparoscopic pancreatic surgery, however, has been slow to gain momentum. Since the first description of minimal access cases reported in 1994 (5), the proportion of laparoscopic pancreatic resections remains low: according to the US Nationwide Inpatient Sample database from 2000 to 2011, only 5% of all resections were performed via a minimal access approach (6). However, with progress in laparoscopic equipment, increasing numbers of cases have been reported in all indications (6,7). Our aim was to review the literature concerning the major advances in minimal access pancreatic surgery.
Definitions
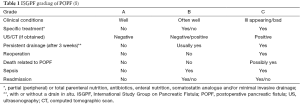
The International Study Group on Pancreatic Fistula (ISGPF) (8) defined postoperative pancreatic fistula (POPF) as “drain output of any measurable volume of fluid on or after postoperative day 3 with amylase content greater than 3 times the serum amylase activity”. Severity is graded from A to C (Table 1).
Distal pancreatectomy (DP)
DP accounts for about a third of all pancreatic resections (6). Indications include benign, pre-malignant and malignant lesions of the pancreatic body/tail such as chronic pancreatitis, endocrine tumors, intraductal papillary mucinous neoplasm (IPNM), pancreatic pseudocysts, mucinous and serous cystic neoplasia, metastases and also trauma with ductal injury (9-11).
MAS accounts for between 10.8% to 46.6% of DP (9,12,13). Several publications have found no statistically significant difference in operative times between laparoscopic DP (LDP) and open DP (ODP), ranging from 156 to 383 min and from 145 to 330 min in laparoscopic and open surgery, respectively (14-17). Conversion rate ranged from 0% to 34% (18,19), hemorrhage and failure to progress being the most common causes. Estimated intraoperative blood loss was found to be significantly lower in LDP (9,13,14,20,21).
Morbidity in LDP has been reported to range from 0% to 67% in single center studies (22,23). However, recent meta-analyses (9,18) described overall morbidity ranging from 34.0% to 37.4%. As morbidity is essentially related to POPF, one possible explanation for this wide range of morbidity may be the use of different definitions for POPF. Adhering to the ISGPF definition, systematic reviews have described the POPF incidence to range from 16.8% to 21.7% in LDP (9,11).
Similarly, reported mortality (range, 0.2–0.4%) (9,18) and reoperation rates (range, 2.1–6.0%) (18,24,25) did not differ from outcomes after open surgery. In spite of a variety of closure techniques available (suture, stapler, sealant, mesh), at the present time there is no proof that one closure technique is better than the other (26-30). Spleen preserving LDP has been described to be safe and feasible (10,22) and has been reported in 18.2% (16) to 60.4% (31) of LDPs.
In their 2015 meta-analysis of 34 studies, Mehrabi et al. (9) described a statistically significant difference in time to first oral intake (0–1.3 days) and duration of stay (DOS) (0–3.8 days). Of note, DOS after DP seemed to be shorter in the United States compared to centers outside of the United States, which might be attributed to differences in health care systems (18). More recently, Shin and colleagues (12) confirmed these reductions in their single center, propensity matched analysis.
Resection margin status was also studied in the meta-analysis by Mehrabi et al. (9): four studies (32-35) reported comparable R0 rates in both groups (592 patients) (OR: 1.63; 95% CI: 0.65–4.07; P=0.29), while the rate of R1 resections was lower in the LDP group (520 patients) (OR: 0.34; 95% CI: 0.14–0.83; P=0.02) (19,34-36).
The mean number of lymph nodes harvested did not differ significantly between LDP and ODP (12 to 13.8 LDP vs. 10 to 12.5 ODP) (12,13). However, the median number (10, range, 1–64) of lymph nodes harvested in the ODP group in one report (12) was less than 12, the recommended number for adequate disease staging (37).
Shin et al. observed a median postoperative survival of 33.4 months in LDP vs. 29.1 months in OPD (P=0.025) (12). In contrast a multicenter study by Kooby et al. found considerably shorter survival (16 months) in both groups (13). While low long-term survival rates are typical for pancreatic cancer, the difference in survival between these last two studies might be attributed to the differences in median tumor size (3.0 vs. 3.5 cm) as well as the type of (monocenter vs. multicenter) study.
Pancreatoduodenectomy (PD)
Due to the anatomical position in the retroperitoneal space, the vicinity to large vessels and the need for three critical anastomoses, PD is considered one of the most challenging operations in GI surgery. Laparoscopic pancreatoduodenectomy (LPD) was described first by Gagner et al. over 20 years ago (5), but since then has not gained widespread use, as it was considered even more difficult (vs. the open approach) with questionable benefits to patients (38). However, with the advance of laparoscopic techniques and improved equipment, the number of LPD performed is continuously rising, as demonstrated by an increase of 50% from 2000 to 2010 according to Tran and colleagues (39).
Several studies have attempted to compare the operative and oncologic characteristics of open and laparoscopic pancreatic head resections, but none were randomized (38-52). Mean operative times have been reported to be significantly longer in LPD, ranging from 452 to 541 min for LPD compared to 372 to 401 min in OPD (40-42), although one center reported non-significant differences (465±86 vs. 465±98 min, respectively) (43). On the other hand, similar to what was observed in LDP, intraoperative blood loss has been reported to be significantly lower in LPD (492.4±519.3 to 841.8±994.8 mL in LPD vs. 866.7±733.7 to 1,452.1±1,966.7 mL in OPD) (43-45). DOS was significantly shorter in several comparative studies (6 to 8 vs. 9 to 12.4 days, respectively) (40,43,44) whereas other studies (7,41) found no statistically significant difference. Conversion to open surgery was reported in 9.1% to 30.0% of cases, mostly due to venous invasion and intraoperative bleeding (7,44,46). Overall morbidity in LPD has been reported to range from 35–52%, however this difference was not found to be statistically significant between the surgical approaches (43,47,48).
Postoperative mortality was recorded to range from 3.2% to 8.8% in LPD vs. 3.4% to 5.7% in OPD, difference which was not statistical significant (7,39,40,43). The reported incidences of clinical relevant POPF (grades B and C) described in several studies were fairly similar, ranging from 6.3% to 11.0% (45,49) in LPD and 5% to 9% (40,43) in open surgery. In their systematic review, Correa-Gallego et al. (44) described overall POPF rates of 21% (8% grade B and C) in LDP and 17% (7% grade B and C) in ODP. This is comparable with Boggi and colleagues (46), who found a 24.8% incidence (10.5% grade B and C) for POPF after LDP in their meta-analysis.
Given that the majority of PDs are performed for malignant or premalignant lesions (7,46,49), adequate oncological resection remains one of the key questions. The number of lymph nodes harvested has been reported to be similar (7,45) or even significantly higher in LPD (40,43,44) compared to PD. Comparisons of R0 resection rates showed that results between open and LPD did not differ significantly (7,40,43,45,50). Of note, however, margin status may not be the ideal parameter for comparisons because definitions of margin involvement vary and under-reporting of microscopic margin involvement has been described (51). Portal venous infiltration as such is not a contraindication for the LPD (52). Interestingly, Croome and colleagues (45) reported a significantly longer interval of progression free survival and a shorter median time to adjuvant chemotherapy in LPD. However, overall survival was not improved, consistent with what is generally observed in pancreatic cancer (43,49).
However, most results come from highly experienced centers for LPD and may not be generally applicable. Moreover, several studies (39,47,48) have indicated that the learning curve is steep, DOS is increased and total costs are higher in centers performing fewer PDs. According to Adam and colleagues in their analysis of 7,061 PD for cancer in the US from 2010–2011, 92% of LPD (14% of all PDs) were undertaken in hospitals performing 10 LPD or less over a 2-year period. They also found a significantly higher 30-day mortality rate in LPD compared to OPD, which was inversely correlated with the volume of LPD per hospital (7). This is in agreement with the OPD learning curves described by Tseng et al. (53) identifying a number of 60 interventions necessary for adequate experience.
Total pancreatectomy (TP)
TP is rarely performed, accounting for 5.4% to 6.7% of all pancreatic resections in high volume centers (54,55).
This may explain why only a few papers (56-60) with small numbers have been published on laparoscopic total pancreatectomy, and thus showing only that it was feasible and safe with apparently satisfactory oncologic outcome.
Parenchyma sparing resections
Parenchyma-sparing resections are indicated in small—benign or low grade malignant—lesions, thus reducing the risk of exocrine and endocrine insufficiency (61). Safety and feasibility of enucleation (EN) and middle pancreatectomy, the most common procedures performed laparoscopically, have been described (62-64).
Indications for parenchyma sparing approaches include mainly neuroendocrine neoplasms, serous cystadenoma and branch duct IPMN as well as solitary renal cell carcinoma metastasis (62,65,66). Depending on the location, tumor size should not exceed 3 to 4 cm in diameter for laparoscopic EN (67,68). Although EN does not include a reconstructive phase, the procedure is associated with a high risk for POPF. In their systematic review on 811 patients undergoing EN, Beger et al. found a 36.7% POPF rate, 16.3% of which were clinically relevant (ISGPF grades B and C) (64). A resection margin equal or less than 2 mm from the main pancreatic duct has been identified as a high risk factor for development of POPF (67).
Zhang and colleagues (61) found no difference between open and laparoscopic EN concerning preservation of pancreatic function, but described shorter operation time as well as lower intraoperative blood loss and faster recovery (in terms of time to first flatus and first oral intake; DOS) in the minimal access approach. A systematic review by Briggs et al. reported conversion rates ranging from 10.5% to 44.4% with a 29.3% POPF rate (31).
Robotic-assisted surgery
The first robotic-assisted pancreatic resections were reported in 2003 by Melvin et al. (69) for DP and by Giulianotti et al. (70) for PD. Since then several reports (71-73) have shown promising results, comparable to and at times better (conversion rate, DOS) than standard laparoscopy and open procedures. While most studies represent early experiences, there is a significant learning curve for robotic pancreatic surgery (74), as in other robotic-assisted procedures (75). Boone et al. (76) described a continuous learning effect with statistically significant improvement after 20 (conversion rate, blood loss), 40 (POPF incidence) and 80 (operative time) procedures.
Of note, the total cost per operation is higher in the robotic approach [$8,304 robotic DP (RDP) vs. $3,861 LDP; robotic PD +€6,200 vs. OPD] (77,78). Interestingly, however, in their single institution experience, Waters et al. (36) reported lower overall costs for robotic DP after adjusting for DOS ($10,588 RDP vs. $12,986 LDP vs. $16,059 ODP). Notwithstanding, hospital costs are most likely subject to substantial variations depending on different health care systems (79).
Conclusions
Laparoscopic pancreatic resections have been shown to be feasible and safe, with rising numbers being reported during the last decade. Most LPD have been performed in university and urban teaching hospitals, while DP seems to be more widely implemented (6).
Comparisons with open surgery have shown reductions in hospital stay and intraoperative blood loss as well as similar results in terms of oncological adequacy. However, none of the data included in this review derive from controlled randomized studies and often represent single center or even single surgeon’s experience, thus underscoring a significant risk for bias. This stresses the need for RCTs wherever possible.
Another major issue is the steep learning curve associated with pancreatic surgery in general and specifically the minimal access approach. Low volume hospitals have been shown to be significantly associated with worse patient outcomes. Robotic assisted surgery is gaining popularity especially in the U.S.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Küper MA, Eisner F, Königsrainer A, et al. Laparoscopic surgery for benign and malign diseases of the digestive system: indications, limitations, and evidence. World J Gastroenterol 2014;20:4883-91. [Crossref] [PubMed]
- Colon Cancer Laparoscopic or Open Resection Study Group, Buunen M, Veldkamp R, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 2009;10:44-52. [Crossref] [PubMed]
- Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050-9. [Crossref] [PubMed]
- Choi YY, Bae JM, An JY, et al. Laparoscopic gastrectomy for advanced gastric cancer: are the long-term results comparable with conventional open gastrectomy? A systematic review and meta-analysis. J Surg Oncol 2013;108:550-6. [Crossref] [PubMed]
- Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408-10. [Crossref] [PubMed]
- Ejaz A, Sachs T, He J, et al. A comparison of open and minimally invasive surgery for hepatic and pancreatic resections using the Nationwide Inpatient Sample. Surgery 2014;156:538-47. [Crossref] [PubMed]
- Adam MA, Choudhury K, Dinan MA, et al. Minimally Invasive Versus Open Pancreaticoduodenectomy for Cancer: Practice Patterns and Short-term Outcomes Among 7061 Patients. Ann Surg 2015;262:372-7. [Crossref] [PubMed]
- Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13. [Crossref] [PubMed]
- Mehrabi A, Hafezi M, Arvin J, et al. A systematic review and meta-analysis of laparoscopic versus open distal pancreatectomy for benign and malignant lesions of the pancreas: it's time to randomize. Surgery 2015;157:45-55. [Crossref] [PubMed]
- Uranues S, Alimoglu O, Todoric B, et al. Laparoscopic resection of the pancreatic tail with splenic preservation. Am J Surg 2006;192:257-61. [Crossref] [PubMed]
- Goh BK, Tan YM, Chung YF, et al. Critical appraisal of 232 consecutive distal pancreatectomies with emphasis on risk factors, outcome, and management of the postoperative pancreatic fistula: a 21-year experience at a single institution. Arch Surg 2008;143:956-65. [Crossref] [PubMed]
- Shin SH, Kim SC, Song KB, et al. A comparative study of laparoscopic vs. open distal pancreatectomy for left-sided ductal adenocarcinoma: a propensity score-matched analysis. J Am Coll Surg 2015;220:177-85. [Crossref] [PubMed]
- Kooby DA, Hawkins WG, Schmidt CM, et al. A multicenter analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate? J Am Coll Surg 2010;210:779-85, 786-7. [Crossref] [PubMed]
- Finan KR, Cannon EE, Kim EJ, et al. Laparoscopic and open distal pancreatectomy: a comparison of outcomes. Am Surg 2009;75:671-9; discussion 679-80. [PubMed]
- Soh YF, Kow AW, Wong KY, et al. Perioperative outcomes of laparoscopic and open distal pancreatectomy: our institution's 5-year experience. Asian J Surg 2012;35:29-36. [Crossref] [PubMed]
- Casadei R, Ricci C, D'Ambra M, et al. Laparoscopic versus open distal pancreatectomy in pancreatic tumours: a case-control study. Updates Surg 2010;62:171-4. [Crossref] [PubMed]
- Tseng WH, Canter RJ, Bold RJ. Perioperative outcomes for open distal pancreatectomy: current benchmarks for comparison. J Gastrointest Surg 2011;15:2053-8. [Crossref] [PubMed]
- Borja-Cacho D, Al-Refaie WB, Vickers SM, et al. Laparoscopic distal pancreatectomy. J Am Coll Surg 2009;209:758-65. [Crossref] [PubMed]
- DiNorcia J, Schrope BA, Lee MK, et al. Laparoscopic distal pancreatectomy offers shorter hospital stays with fewer complications. J Gastrointest Surg 2010;14:1804-12. [Crossref] [PubMed]
- Baker MS, Bentrem DJ, Ujiki MB, et al. A prospective single institution comparison of peri-operative outcomes for laparoscopic and open distal pancreatectomy. Surgery 2009;146:635-43; discussion 643-5. [Crossref] [PubMed]
- Lee SY, Allen PJ, Sadot E, et al. Distal pancreatectomy: a single institution's experience in open, laparoscopic, and robotic approaches. J Am Coll Surg 2015;220:18-27. [Crossref] [PubMed]
- Pryor A, Means JR, Pappas TN. Laparoscopic distal pancreatectomy with splenic preservation. Surg Endosc 2007;21:2326-30. [Crossref] [PubMed]
- Nakamura Y, Uchida E, Aimoto T, et al. Clinical outcome of laparoscopic distal pancreatectomy. J Hepatobiliary Pancreat Surg 2009;16:35-41. [Crossref] [PubMed]
- Venkat R, Edil BH, Schulick RD, et al. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg 2012;255:1048-59. [Crossref] [PubMed]
- Lillemoe KD, Kaushal S, Cameron JL, et al. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg 1999;229:693-8; discussion 698-700. [Crossref] [PubMed]
- Zhou W, Lv R, Wang X, et al. Stapler vs suture closure of pancreatic remnant after distal pancreatectomy: a meta-analysis. Am J Surg 2010;200:529-36. [Crossref] [PubMed]
- Smits FJ, van Santvoort HC, Besselink MG, et al. Systematic review on the use of matrix-bound sealants in pancreatic resection. HPB (Oxford) 2015;17:1033-9. [Crossref] [PubMed]
- Diener MK, Seiler CM, Rossion I, et al. Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): a randomised, controlled multicentre trial. Lancet 2011;377:1514-22. [Crossref] [PubMed]
- Kawai M, Hirono S, Okada KI, et al. Randomized Controlled Trial of Pancreaticojejunostomy versus Stapler Closure of the Pancreatic Stump During Distal Pancreatectomy to Reduce Pancreatic Fistula. Ann Surg 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Park JS, Lee DH, Jang JY, et al. Use of TachoSil(®) patches to prevent pancreatic leaks after distal pancreatectomy: a prospective, multicenter, randomized controlled study. J Hepatobiliary Pancreat Sci 2016;23:110-7. [Crossref] [PubMed]
- Briggs CD, Mann CD, Irving GR, et al. Systematic review of minimally invasive pancreatic resection. J Gastrointest Surg 2009;13:1129-37. [Crossref] [PubMed]
- Kang CM, Kim DH, Lee WJ. Ten years of experience with resection of left-sided pancreatic ductal adenocarcinoma: evolution and initial experience to a laparoscopic approach. Surg Endosc 2010;24:1533-41. [Crossref] [PubMed]
- Jayaraman S, Gonen M, Brennan MF, et al. Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg 2010;211:503-9. [Crossref] [PubMed]
- Stauffer JA, Rosales-Velderrain A, Goldberg RF, et al. Comparison of open with laparoscopic distal pancreatectomy: a single institution's transition over a 7-year period. HPB (Oxford) 2013;15:149-55. [Crossref] [PubMed]
- Limongelli P, Belli A, Russo G, et al. Laparoscopic and open surgical treatment of left-sided pancreatic lesions: clinical outcomes and cost-effectiveness analysis. Surg Endosc 2012;26:1830-6. [Crossref] [PubMed]
- Waters JA, Canal DF, Wiebke EA, et al. Robotic distal pancreatectomy: cost effective? Surgery 2010;148:814-23. [Crossref] [PubMed]
- Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol 2008;15:165-74. [Crossref] [PubMed]
- Gagner M, Pomp A. Laparoscopic pancreatic resection: Is it worthwhile? J Gastrointest Surg 1997;1:20-5; discussion 25-6. [Crossref] [PubMed]
- Tran TB, Dua MM, Worhunsky DJ, et al. The First Decade of Laparoscopic Pancreaticoduodenectomy in the United States: Costs and Outcomes Using the Nationwide Inpatient Sample. Surg Endosc 2015. [Epub ahead of print]. [PubMed]
- Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg 2012;215:810-9. [Crossref] [PubMed]
- Zureikat AH, Breaux JA, Steel JL, et al. Can laparoscopic pancreaticoduodenectomy be safely implemented? J Gastrointest Surg 2011;15:1151-7. [Crossref] [PubMed]
- Merkow J, Paniccia A, Edil BH. Laparoscopic pancreaticoduodenectomy: a descriptive and comparative review. Chin J Cancer Res 2015;27:368-75. [PubMed]
- Croome KP, Farnell MB, Que FG, et al. Pancreaticoduodenectomy with major vascular resection: a comparison of laparoscopic versus open approaches. J Gastrointest Surg 2015;19:189-94; discussion 194. [Crossref] [PubMed]
- Correa-Gallego C, Dinkelspiel HE, Sulimanoff I, et al. Minimally-invasive vs open pancreaticoduodenectomy: systematic review and meta-analysis. J Am Coll Surg 2014;218:129-39. [Crossref] [PubMed]
- Croome KP, Farnell MB, Que FG, et al. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg 2014;260:633-8; discussion 638-40. [Crossref] [PubMed]
- Boggi U, Amorese G, Vistoli F, et al. Laparoscopic pancreaticoduodenectomy: a systematic literature review. Surg Endosc 2015;29:9-23. [Crossref] [PubMed]
- Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg 2015;220:530-6. [Crossref] [PubMed]
- Schmidt CM, Turrini O, Parikh P, et al. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: a single-institution experience. Arch Surg 2010;145:634-40. [Crossref] [PubMed]
- Song KB, Kim SC, Hwang DW, et al. Matched Case-Control Analysis Comparing Laparoscopic and Open Pylorus-preserving Pancreaticoduodenectomy in Patients With Periampullary Tumors. Ann Surg 2015;262:146-55. [Crossref] [PubMed]
- Dokmak S, Ftériche FS, Aussilhou B, et al. Laparoscopic pancreaticoduodenectomy should not be routine for resection of periampullary tumors. J Am Coll Surg 2015;220:831-8. [Crossref] [PubMed]
- Verbeke CS, Menon KV. Redefining resection margin status in pancreatic cancer. HPB (Oxford) 2009;11:282-9. [Crossref] [PubMed]
- Khatkov IE, Izrailov RE, Tiutiunnik PS, et al. Totally Laparoscopic Pancreatico-Duodenectomy with Tangential Portal Vein Resection. Eur J Endosc Laparosc Surg 2014;1:92-5.
- Tseng JF, Pisters PW, Lee JE, et al. The learning curve in pancreatic surgery. Surgery 2007;141:694-701. [Crossref] [PubMed]
- Crippa S, Tamburrino D, Partelli S, et al. Total pancreatectomy: indications, different timing, and perioperative and long-term outcomes. Surgery 2011;149:79-86. [Crossref] [PubMed]
- Janot MS, Belyaev O, Kersting S, et al. Indications and early outcomes for total pancreatectomy at a high-volume pancreas center. HPB Surg 2010;2010.
- Dallemagne B, de Oliveira AT, Lacerda CF, et al. Full laparoscopic total pancreatectomy with and without spleen and pylorus preservation: a feasibility report. J Hepatobiliary Pancreat Sci 2013;20:647-53. [Crossref] [PubMed]
- Giulianotti PC, Addeo P, Buchs NC, et al. Early experience with robotic total pancreatectomy. Pancreas 2011;40:311-3. [Crossref] [PubMed]
- Casadei R, Marchegiani G, Laterza M, et al. Total pancreatectomy: doing it with a mini-invasive approach. JOP 2009;10:328-31. [PubMed]
- Galvani CA, Rodriguez Rilo H, Samamé J, et al. Fully robotic-assisted technique for total pancreatectomy with an autologous islet transplant in chronic pancreatitis patients: results of a first series. J Am Coll Surg 2014;218:e73-8. [Crossref] [PubMed]
- Choi SH, Hwang HK, Kang CM, et al. Pylorus- and spleen-preserving total pancreatoduodenectomy with resection of both whole splenic vessels: feasibility and laparoscopic application to intraductal papillary mucin-producing tumors of the pancreas. Surg Endosc 2012;26:2072-7. [Crossref] [PubMed]
- Zhang T, Xu J, Wang T, et al. Enucleation of pancreatic lesions: indications, outcomes, and risk factors for clinical pancreatic fistula. J Gastrointest Surg 2013;17:2099-104. [Crossref] [PubMed]
- Crippa S, Bassi C, Warshaw AL, et al. Middle pancreatectomy: indications, short- and long-term operative outcomes. Ann Surg 2007;246:69-76. [Crossref] [PubMed]
- Fernández-Cruz L, Molina V, Vallejos R, et al. Outcome after laparoscopic enucleation for non-functional neuroendocrine pancreatic tumours. HPB (Oxford) 2012;14:171-6. [Crossref] [PubMed]
- Beger HG, Siech M, Poch B, et al. Limited surgery for benign tumours of the pancreas: a systematic review. World J Surg 2015;39:1557-66. [Crossref] [PubMed]
- Kuroki T, Eguchi S. Laparoscopic parenchyma-sparing pancreatectomy. J Hepatobiliary Pancreat Sci 2014;21:323-7. [Crossref] [PubMed]
- Damoli I, Butturini G, Ramera M, et al. Minimally invasive pancreatic surgery - a review. Wideochir Inne Tech Maloinwazyjne 2015;10:141-9. [Crossref] [PubMed]
- Brient C, Regenet N, Sulpice L, et al. Risk factors for postoperative pancreatic fistulization subsequent to enucleation. J Gastrointest Surg 2012;16:1883-7. [Crossref] [PubMed]
- Pitt SC, Pitt HA, Baker MS, et al. Small pancreatic and periampullary neuroendocrine tumors: resect or enucleate? J Gastrointest Surg 2009;13:1692-8. [Crossref] [PubMed]
- Melvin WS, Needleman BJ, Krause KR, et al. Robotic resection of pancreatic neuroendocrine tumor. J Laparoendosc Adv Surg Tech A 2003;13:33-6. [Crossref] [PubMed]
- Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003;138:777-84. [Crossref] [PubMed]
- Daouadi M, Zureikat AH, Zenati MS, et al. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg 2013;257:128-32. [Crossref] [PubMed]
- Boggi U, Palladino S, Massimetti G, et al. Laparoscopic robot-assisted versus open total pancreatectomy: a case-matched study. Surg Endosc 2015;29:1425-32. [Crossref] [PubMed]
- Zureikat AH, Nguyen T, Boone BA, et al. Robotic total pancreatectomy with or without autologous islet cell transplantation: replication of an open technique through a minimal access approach. Surg Endosc 2015;29:176-83. [Crossref] [PubMed]
- Strijker M, van Santvoort HC, Besselink MG, et al. Robot-assisted pancreatic surgery: a systematic review of the literature. HPB (Oxford) 2013;15:1-10. [Crossref] [PubMed]
- Hayn MH, Hussain A, Mansour AM, et al. The learning curve of robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Eur Urol 2010;58:197-202. [Crossref] [PubMed]
- Boone BA, Zenati M, Hogg ME, et al. Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg 2015;150:416-22. [Crossref] [PubMed]
- Boggi U, Signori S, De Lio N, et al. Feasibility of robotic pancreaticoduodenectomy. Br J Surg 2013;100:917-25. [Crossref] [PubMed]
- Kang CM, Kim DH, Lee WJ, et al. Conventional laparoscopic and robot-assisted spleen-preserving pancreatectomy: does da Vinci have clinical advantages? Surg Endosc 2011;25:2004-9. [Crossref] [PubMed]
- Ridic G, Gleason S, Ridic O. Comparisons of health care systems in the United States, Germany and Canada. Mater Sociomed 2012;24:112-20. [Crossref] [PubMed]
Cite this article as: Justin V, Fingerhut A, Khatkov I, Uranues S. Laparoscopic pancreatic resection—a review. Transl Gastroenterol Hepatol 2016;1:36.