Study on laparoscopic spleen preserving distal pancreatectomy procedures comparing splenic vessel preservation and non-preservation
Introduction
Over the past few years, there have been an increasing number of reports on the feasibility of laparoscopic distal pancreatectomy (Lap-DP) (1-7). Some meta-analyses have compared Lap-DP and open distal pancreatectomy and all have indicated that Lap-DP is superior due to less invasiveness (8-13). Today, Lap-DP is recognized worldwide as a feasible and highly beneficial procedure.
Theoretically, preservation of the spleen is preferred, especially in patients with the benign diseases and/or low grade malignant tumors, simply due to its favorable role in regulating balance between the hematologic and immune systems (14,15). There are two methods in spleen preserving distal pancreatectomy (SPDP), one is splenic vessels preserving SPDP (SPDP-VP) or another is the Warshaw operation (16). However, further clarification of postoperative results of the laparoscopic SPDP (Lap-SPDP) for the patients with benign or low grade malignant diseases is needed.
The purpose of this study is to investigate whether two types of Lap-SPDP techniques are being implemented safely. The study compares the clinical outcomes from laparoscopic Warshaw operation (Lap-W) with those from laparoscopic SPDP-VP (Lap-SPDP-VP) and considers the role of those operations.
Study design
The study was a retrospective, comparative study over a limited time frame.
Materials and methods
We previously reported the clinical data of 100 patients who underwent Lap-DP between January 2004 (after Lap-DP was approved by the hospital’s Ethics Review Board of Nippon Medical School) and December 2013, including data of Lap-SPDP-VP (17). On August 2013, the Warshaw technique was introduced to our institution and 17 patients with a lesion in the distal pancreas who underwent Lap-SPDP by December 2015 were enrolled. Six of the 17 patients underwent Lap-W and the remaining 11 patients underwent Lap-SPDP-VP. The first author (Yoshiharu Nakamura) participated in all operations as an operator or technical coach. We retrospectively compared both the perioperative outcomes and late-phase follow-up data between the Lap-W group and Lap-SPDP-VP group which underwent surgery during the same period (from August 2013 to December 2015). Written informed consent was received from all 17 patients undergoing Lap-SPDP.
Lap-SPDP which could preserve splenic vessels was first selected for all proposed SPDP cases. However, since August 2013, for cases where this was not possible due to technical reasons or unsuitability for curable resection, strategy was changed to use either a laparoscopic spleen sacrificed distal pancreatectomy or a procedure which used Lap-W.
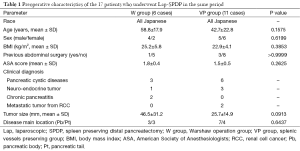
All patients were Japanese. Average age was 58.8 years in the Lap-W group (W group) and 42.7 years in the Lap-SPDP-VP group (VP group). The W group consisted of four males and two females with an average body mass index (BMI) of 25.2 kg/m2. The VP group consisted of five males and six females with an average BMI of 22.9 kg/m2. One of the six patients (16.7%) in the W group had a history of abdominal surgery, while three of the 11 patients (27.3%) in the VP group had a history of abdominal surgery. Mean American Society of Anesthesiologists physical status score [American Society of Anesthesiologists (ASA) score] was 1.8 in the W group and 1.5 in the VP group. Final diagnosis was based on either intraoperative frozen section pathological diagnosis or postoperative histopathological diagnosis. Diagnosis of pancreatic cystic disease was made for three patients in the W group [mucinous cystic neoplasm (MCN) (n=1), solid pseudopapillary neoplasm (n=1), serous cystic neoplasm (SCN) (n=1)] and for six patients in the VP group [solid pseudopapillary neoplasm (n=5), minimally invasive cancer derived from intraductal papillary mucinous neoplasm (IPMN) (n=1)]. Diagnosis of neuroendocrine tumor was made for one patient in the W group [non-functioning tumor (n=1)] and for 3 patients in the VP group [non-functioning tumor (n=2), insulinoma (n=1)]. Diagnosis of metastatic tumor from renal cell cancer (RCC) was made for two patients in the VP group.
Mean tumor size was 46.5 mm in the W group and 25.7 mm in the VP group. In the W group, tumors were mainly located in the pancreatic body (Pb) in three patients, and the pancreatic tail (Pt) in three patients. In the VP group, tumors were mainly located in the Pb in seven patients, Pt in four patients (Table 1).
Full table
Pancreatic fistula was defined according to the 2005 International Study Group of Pancreatic Fistula, and clinical leaks were classified into Grades B and C (18,19).
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) version 19.0 software program (SPSS Inc., Chicago, USA). Data were expressed as means ± standard deviation (SD) or the median. Data from each period were compared using an unpaired t-test, Mann-Whitney U-test or Fisher’s exact test, as appropriate. Probability (P) values of less than 0.05 were considered significant.
Operative technique
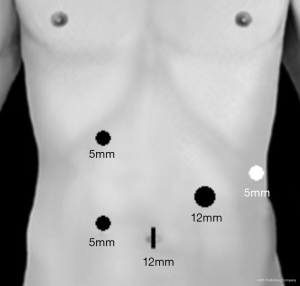
We previously described our technique for Lap-SPDP-VP in a number of English papers (4,17,20). Each patient was immobilized in the supine position and patient angles were adjusted as needed by rotating the operating table. A 12 mm umbilical trocar was used to insert the laparoscope and endoscopic linear stapler (ELS) (End-GIA, Covidien, Norwalk, CT, USA) [(I) 12 mm]. On the right side, a 5 mm trocar was inserted below the right costal arch between the right mammillary line and the upper abdominal median line [(II) 5 mm], and another 5 mm trocar was inserted at 10 cm caudal to (II) [(III) 5 mm]. On the left side, a 5 mm trocar was placed at the anterior subcostal region—midaxillary line [(IV) 5 mm] and a 12 mm trocar was inserted at the center point between (I) and (IV) [(V) 12 mm] (Figure 1). Intra-abdominal air pressure was set at 7–10 mmHg with carbon dioxide.
For Lap-W procedure, the greater omentum is widely divided to expose the anterior side of the Pb and tail, whereas the splenocolic ligament, gastrosplenic ligament and left gastroepiploic vessels are preserved in all SPDP proposed cases, in order to maintain the blood supply to the spleen in cases where Lap-SPDP-VP is switched over to Lap-W. After intraoperative laparoscopic ultrasonography for confirmation of tumor location and for determination of the resection line of the pancreas, in the Lap-W procedure, the splenic artery is mobilized near the planed cut line of the pancreas and divided by clipping. Consequently, the splenic vein is also divided at the same site and an ELS is used to transect the pancreas. Next, distal pancreas dissection from the retroperitoneal space is continued up to the hilum of the spleen. The Pt is detached from the splenic hilum and then splenic vessels were carefully divided so as not to damage the small vessels in the hilum of the spleen. The dissected pancreas is placed in an endoscopic retrieval bag and extracted through the umbilical wound.
Results
Preoperative characteristics including age, sex, BMI, history of abdominal surgery, ASA score, final diagnosis of disease, tumor size, and tumor location between the two groups are comparative (Table 1). Although there was no statistical difference, a tendency for large tumor size was observed in the W group (46.5±31.2 vs. 25.7±14.9 mm; P=0.0913) (Table 1).
Median operating time was 287 min (range, 225–369 min) in the W group and 280 min (range, 200–496 min) in the VP group. No significant difference was observed between groups (P=0.9112). Median blood loss was 95 mL (range, 50–200 mL) for the W group and 60 mL (range, 0–650 mL) for the VP group. No significant difference was observed between groups (P=0.5502). No cases underwent blood transfusion, combined operation, or hand-assisted procedures in either group. In the VP group, one patient was switched to open SPDP-VP due to bleeding from the splenic vein, and two patients were switched to laparoscopic spleen sacrificed distal pancreatectomy due to accompanying severe associated pancreatitis and derived cancer from IPMN which was proven by intraoperative pathological study (Table 2).

Full table
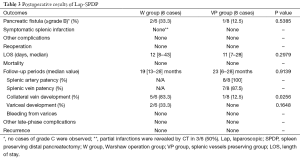
No patient with grade C pancreatic fistulae was observed in either group. Grade B pancreatic fistulae was observed in 33.3% (two of six patients) of patients in the W group and one out of eight patients (12.5%) in the VP group. No significant difference was observed between groups (P=0.5385). Pancreatic fistulae was resolved by conservative treatment for all patients in both groups. Regarding postoperative complications other than pancreatic fistula, no case was observed in either group, including symptomatic spleen infarction. However, partial infarctions of the spleen without symptoms were observed by computed tomography in three out of six cases (50%) in the W group. Median postoperative hospital stay was 12 days (range, 8–43 days) in the W group and 11 days (range, 7–28 days) in the VP group. No significant difference was observed between groups in terms of the postoperative hospital stay (P=0.2079). No patient required reoperation and the postoperative mortality was zero in both groups (Table 3).
Full table
Median follow-up was 19 months (range, 13–28 months) for the W group and 23 months (range, 6–28 months) for the VP group, and no significant difference was observed between groups (P=0.9139). All patients were alive and recurrence-free at the end of the follow-up period. In the VP group, patency of splenic artery was saved in 100% of cases and patency of splenic vein was saved in seven out of eight patients (87.5%). Collateral veins around the spleen developed in 83.3% (five out of six patients) in the W group and developed in 12.5% (one out of eight patients) in the VP group. A significant difference was observed between groups (P=0.0256). Gastric varices developed in 33.3% (two out of six patients) in the W group and developed in 0% of the cases in the VP group. No significant difference was observed between groups (P=0.1648). However, no case of rupture of varices, or other late phase complications was observed in either group (Table 3).
Discussion
Laparoscopic surgery is now widely performed because (I) the surgical wound is small and outcome is cosmetically favorable; (II) areas not easily macroscopically visible can be clearly visualized using a laparoscope, allowing more detailed procedures and reducing blood loss; and (III) minimal surgical invasiveness leads to a shorter postoperative hospital stay, thus lowering costs. These advantages also apply to the use of laparoscopic surgery to treat pancreatic diseases.
Theoretically, preservation of the spleen is preferred, especially in patients with low grade malignant diseases, simply due its favorable role in regulating the balance between the hematologic and immune systems (14,15). The Warshaw operation was first described in 1988. Splenic vessels are resected and the spleen survives via the short gastric and left gastroepiploic vessels (16). Ferrone et al. described their cases of the Warshaw operation, focusing on possible long-term complications between February 1986 and February 2009 (21). Out of 158 cases who underwent Warshaw operation, only three (1.9%) patients required reoperation for splenic infarction 3–100 days postoperatively due to abdominal pain and/or fever. Median follow-up was 2.7 years (mean, 4.5 years, range, 0–21 years). Evidence of perigastric varices was observed in 16 out of 65 (25%) patients who received follow-up imaging at a median time period of 3.4 years, but none of the 158 patients developed gastrointestinal bleeding or hypersplenism (21).
Matsushima et al. reported on the operative outcomes of 17 cases that underwent Lap-W. Splenic infarctions were observed in four patients (24%) and perigastric varices were observed in two patients (12%) (22). All these patients were observed conservatively. Matsushima described the importance of preserving not only the short gastric vessels but also the splenocolic ligament and left gastroepiploic vessels in order to retain the blood supply to the spleen in the Warshaw operation (23), and considered that the laparoscopic method of the Warshaw operation may be a more feasible way to preserve the blood supply to the spleen, since a laparoscopic technique can visualize thinner vessels and can therefore avoid unnecessary vessel treatment (22).
At this time, we comparatively considered the achievements of Lap-W and Lap-SPDP-VP, since the introduction of Lap-W. No significant difference in perioperative results was observed. Regarding long-term achievement, the occurrence rate of patency of the splenic vessels after switching to Lap-SPDP-VP was in agreement as that reported in previous literature (24,25). Although the sample size of Lap-W cases was small at this time, collateral vein development was significantly large, and even variceal development was observed in 33% of the cases. Cases of bleeding from gastric varices during splenic artery and vein excision in Open-SPDP have been reported (26). Furthermore, in the Lap-SPDP-VP group, development of collateral veins was observed one out of eight patients. While SPDP is recommended for patients with benign or low grade malignant diseases, long-term follow-up to monitor hemodynamic changes in splenogastric circulation is considered needed.
In our results, a higher tendency for large tumor size was observed in the W group. In such cases, it is very difficult to detach the splenic vessels from the tumor or the pancreatic parenchyma. Detachment work in these cases is even more difficult in laparoscopic surgery. Similarly, detachment in cases complicated by associated pancreatitis are difficult. Furthermore, even in cases of low grade malignancy, the tumor capsule may remain when detaching the splenic vessels, hence complete cure is considered difficult. In such cases, performing Lap-W, rather than Lap-SPDP-VP, is considered appropriate.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional ethical committee. Written informed consent was obtained from the patient for publication. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Kooby DA, Gillespie T, Bentrem D, et al. Left-sided pancreatectomy: a multicenter comparison of laparoscopic and open approaches. Ann Surg 2008;248:438-46. [PubMed]
- Kim SC, Park KT, Hwang JW, et al. Comparative analysis of clinical outcomes for laparoscopic distal pancreatic resection and open distal pancreatic resection at a single institution. Surg Endosc 2008;22:2261-8. [Crossref] [PubMed]
- Baker MS, Bentrem DJ, Ujiki MB, et al. A prospective single institution comparison of peri-operative outcomes for laparoscopic and open distal pancreatectomy. Surgery 2009;146:635-43; discussion 643-5. [Crossref] [PubMed]
- Nakamura Y, Uchida E, Aimoto T, et al. Clinical outcome of laparoscopic distal pancreatectomy. J Hepatobiliary Pancreat Surg 2009;16:35-41. [Crossref] [PubMed]
- Jayaraman S, Gonen M, Brennan MF, et al. Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg 2010;211:503-9. [Crossref] [PubMed]
- Vijan SS, Ahmed KA, Harmsen WS, et al. Laparoscopic vs open distal pancreatectomy: a single-institution comparative study. Arch Surg 2010;145:616-21. [Crossref] [PubMed]
- Butturini G, Inama M, Malleo G, et al. Perioperative and long-term results of laparoscopic spleen-preserving distal pancreatectomy with or without splenic vessels conservation: a retrospective analysis. J Surg Oncol 2012;105:387-92. [Crossref] [PubMed]
- Venkat R, Edil BH, Schulick RD, et al. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg 2012;255:1048-59. [Crossref] [PubMed]
- Sui CJ, Li B, Yang JM, et al. Laparoscopic versus open distal pancreatectomy: a meta-analysis. Asian J Surg 2012;35:1-8. [Crossref] [PubMed]
- Jin T, Altaf K, Xiong JJ, et al. A systematic review and meta-analysis of studies comparing laparoscopic and open distal pancreatectomy. HPB (Oxford) 2012;14:711-24. [Crossref] [PubMed]
- Xie K, Zhu YP, Xu XW, et al. Laparoscopic distal pancreatectomy is as safe and feasible as open procedure: a meta-analysis. World J Gastroenterol 2012;18:1959-67. [Crossref] [PubMed]
- Pericleous S, Middleton N, McKay SC, et al. Systematic review and meta-analysis of case-matched studies comparing open and laparoscopic distal pancreatectomy: is it a safe procedure? Pancreas 2012;41:993-1000. [Crossref] [PubMed]
- Nakamura M, Nakashima H. Laparoscopic distal pancreatectomy and pancreatoduodenectomy: is it worthwhile? A meta-analysis of laparoscopic pancreatectomy. J Hepatobiliary Pancreat Sci 2013;20:421-8. [Crossref] [PubMed]
- Shoup M, Brennan MF, McWhite K, et al. The value of splenic preservation with distal pancreatectomy. Arch Surg 2002;137:164-8. [Crossref] [PubMed]
- Kang CM, Chung YE, Jung MJ, et al. Splenic vein thrombosis and pancreatic fistula after minimally invasive distal pancreatectomy. Br J Surg 2014;101:114-9. [Crossref] [PubMed]
- Warshaw AL. Conservation of the spleen with distal pancreatectomy. Arch Surg 1988;123:550-3. [Crossref] [PubMed]
- Nakamura Y, Matsushita A, Katsuno A, et al. Laparoscopic distal pancreatectomy: Educating surgeons about advanced laparoscopic surgery. Asian J Endosc Surg 2014;7:295-300. [Crossref] [PubMed]
- Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13. [Crossref] [PubMed]
- Pratt W, Maithel SK, Vanounou T, et al. Postoperative pancreatic fistulas are not equivalent after proximal, distal, and central pancreatectomy. J Gastrointest Surg 2006;10:1264-78; discussion 1278-9. [Crossref] [PubMed]
- Nakamura Y, Matsushita A, Katsuno A, et al. Clinical outcomes for 14 consecutive patients with solid pseudopapillary neoplasms who underwent laparoscopic distal pancreatectomy. Asian J Endosc Surg 2016;9:32-6. [Crossref] [PubMed]
- Ferrone CR, Konstantinidis IT, Sahani DV, et al. Twenty-three years of the Warshaw operation for distal pancreatectomy with preservation of the spleen. Ann Surg 2011;253:1136-9. [Crossref] [PubMed]
- Matsushima H, Kuroki T, Adachi T, et al. Laparoscopic spleen-preserving distal pancreatectomy with and without splenic vessel preservation: the role of the Warshaw procedure. Pancreatology 2014;14:530-5. [Crossref] [PubMed]
- Romero-Torres R. The true splenic blood supply and its surgical applications. Hepatogastroenterology 1998;45:885-8. [PubMed]
- Yoon YS, Lee KH, Han HS, et al. Patency of splenic vessels after laparoscopic spleen and splenic vessel-preserving distal pancreatectomy. Br J Surg 2009;96:633-40. [Crossref] [PubMed]
- Hwang HK, Chung YE, Kim KA, et al. Revisiting vascular patency after spleen-preserving laparoscopic distal pancreatectomy with conservation of splenic vessels. Surg Endosc 2012;26:1765-71. [Crossref] [PubMed]
- Miura F, Takada T, Asano T, et al. Hemodynamic changes of splenogastric circulation after spleen-preserving pancreatectomy with excision of splenic artery and vein. Surgery 2005;138:518-22. [Crossref] [PubMed]
Cite this article as: Nakamura Y, Matsushita A, Mizuguchi Y, Katsuno A, Uchida E. Study on laparoscopic spleen preserving distal pancreatectomy procedures comparing splenic vessel preservation and non-preservation. Transl Gastroenterol Hepatol 2016;1:27.


