Multiple localized metachronous recurrences in a patient of colon cancer and therapeutic controversies in stage II colon cancer
Introduction
Our patient, a 57-year-old male, presented to the surgical department in year 1994 with dull aching pain in the lower abdomen and constipation for 3 months. There were several episodes of passing blood in stools for 1 month. He was a chronic smoker (12–15 cigarettes/day for 20 years). There was no family history of malignancy. He was diagnosed to have caecal growth; right hemi-colectomy was done on 27-7-1994. Intra-operatively; caecum showed a polypoidal growth 9×7 cm2, extending up to base of appendix. There was no ascites, liver or peritoneal deposits. Microscopy showed poorly differentiated adenocarcinoma and resection margins were free of tumour. Two lymph nodes dissected showed reactive hyperplasia. In view of poorly differentiated tumour and only two lymph nodes retrieved, patient was started on adjuvant chemotherapy with weekly 5-FU (750 mg) and leucovorin (200 mg). Completed 12 such courses on 22-10-1994. Subsequently he was kept on follow-up with serial colonoscopy and ultrasound abdomen which was normal until the year 2002 when he developed a new growth in the rectum. He underwent laparotomy + excision of the sigmoid + upper 2/3rd of the rectum + low anterior meso-rectal excision + colorectal anastomosis on 01-04-2002. Intra-operatively, there was growth 6×5 cm2 in the upper 1/3 and middle 1/3 of the rectum, no ascites or secondaries in the liver, no pelvic deposits, no enlarged epicolic or paracolic lymph nodes. Microscopy showed adenocarcinoma moderately differentiated adenocarcinoma (Figure 1).
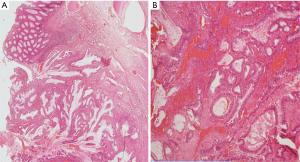
No lympho-vascular space invasion was seen and resection limits were free of tumour. All 13 lymph nodes removed were free of tumour. External Radiation was delivered 40 Gy/20# from 16-5-2002 to 10-6-2002 in view of the tumour being located in the rectum and infiltrating up to serosa. Patient was also given adjuvant chemotherapy with FOLFOX regimen (3 weekly) consisting of Inj Oxaliplatin 135 mg/m2 i.v with 5% dextrose, Inj 5-FU 1.2 g/m2 i.v infusion over 4 hours six cycles of chemotherapy was completed on 20-02-2003. Patient was on follow-up with serial CT scans, colonoscopy and carcino embryonic antigen (CEA), when he presented with repeated episodes of bleeding in the stools. Colonoscopy revealed asymmetrical mural thickening of the descending colon. Patient underwent Total colectomy with ileo-rectal anastomosis on 28-2-2013. Intra-operatively, tumour 5×4 cm2 was palpated in the descending colon; no enlarged lymph nodes or nodules on the surface of the liver/peritoneum or ascites. Histopathology revealed moderately differentiated adenocarcinoma with tumor cells infiltrating transmurally forming serosal nodules (Figure 2).
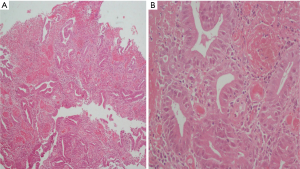
Circumferential, proximal and distal resection limits were free of tumour. Six lymph nodes identified were free of tumour. Patient was started on adjuvant chemotherapy (in view of transmural infiltration of disease); FOLFOX regimen 2 weekly: Oxaliplatin 85 mg/m2 in 5% dextrose, and 5-FU 1.2 g/m2 infusion over 4 hours. Chemotherapy was completed on 05-09-2013. Patient was on regular follow-up, last CT scan done on 14-05-2014 was within normal limits. Patient was last seen by us on 12-08-2014, was asymptomatic and clinically disease free.
Discussion
This case is being reported due to presentation with multiple localized metachronous recurrences in the absence of prior polyposis and family history of colonic cancer. The index case also highlights the survival benefit derived from regular screening with colonoscopy after successful initial therapy. The patient underwent repeated colonoscopies during surveillance in between the recurrences which did not show presence of polyposis or dysplastic changes. All the recurrences presented in stage II. With each of the recurrences the patient required adjuvant therapy for different indications. The management of stage II colonic cancer after curative resection is a controversial subject as robust evidence for and against the use of adjuvant chemotherapy is lacking. Therefore, we review the factors which influence the categorization of patients as high risk of developing recurrence. Rate of recurrence after successful initial therapy in colon cancer is 30–40%. Majority of recurrences (60–65%) are distant—liver, lungs, lymph nodes. Median survival after recurrence is 18–28 months (improving due to newer drugs, targeted agents, and hepatic resections). Prior chemotherapy 5-FU based (due to selection & sensitivity effect), lymph node negative disease (stage II/dukes B) and long latency before recurrence were highly predictive of survival. Patients treated in the modern era after recurrence have a better prognosis Majority of patients with stage II colon cancer are cured by resection, 20–25% relapse, die of the disease and hence the survival is around 70–80%. The role of adjuvant therapies is controversial as evidence supporting or refuting benefit is lacking. This is because, inherent survival after surgical resection is high (70–80%), large patient numbers are required to show further benefit. Nevertheless it is important to identify high risk subsets. NSABP analysis (1,2) showed a 30% reduction in mortality with adjuvant chemotherapy which was criticized for the non-homogeneity of the test groups and non-standard statistical analysis. SEER Analysis which was a population based study failed to show any benefit (3,4). IMPACT meta-analysis from randomized trials showed an absolute risk reduction for treated patients was 3% for 5-year disease-free survival (DFS) and 2% for 5-year overall survival, which was not statistically significant (5). Subset analysis of the mosaic trial suggested no benefit to elderly patients (6). QUASAR trial (7) is the only trial which looked at stage II colon cancer showed a 3.6% absolute survival benefit for this stage. The major bodies like American Society of Clinical Oncology, ACSO and European Society of Medical Oncology (ESMO) have recommended use of chemotherapy in those with high risk features which are elaborated below. Factors predicting high recurrence risk in stage II colon cancer:
- T stage: a worse prognosis for T4N0M0 patients similar to stage III disease i.e., node positive disease (8). pT3 tumors with deep invasion >15 mm beyond muscularis propria have a high risk of recurrence (9);
- Obstructing or perforating tumors: they also have survival rates similar to the stage III patients (INT-0035 results) (10);
- Lymph node retrieval rate: colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089 (11,12). The AJCC and TNM committee recommends a minimum of 12 lymph nodes to perform correct pathological staging. Patients with stage II colon cancer with <12 retrieved nodes should be considered for adjuvant treatment. Other pathological findings like venous invasion, and in particular invasion of extramural veins, seems to be a particularly strong adverse prognostic factor. Mucinous histology, poorly differentiated tumors and perineural invasion are also frequently quoted as ‘negative’ prognostic factors for stage II patients;
- Chromosomal abnormalities: allelic imbalance due to the loss of chromosome arms. When the lost region contains a tumor suppressor gene, cells with this kind of defect can acquire a competitive growth advantage allelic loss of chromosome 18q on the prognosis of stage II colon cancer (13). (Survival: 83% of the patients without 18q allelic loss vs. 58% of patients with 18q allelic loss). Microsatellite instability 5-year survival advantage for patients whose tumors had MSI-H compared to those with microsatellite-stable tumors (74% vs. 46%) (14);
- Other molecular markers: expressions of thymidylate synthetase (TYMS): adjuvant therapy significantly improved survival in patients with high TYMS levels (P=0.04) (15). V600E mutation in the BRAF gene is also a poor prognostic factor for disease free and overall survival (16);
- Age: it is an important factor in decision making particularly especially in those aged above 70 years when average life expectancy is <10 years. All the trials including MOSAIC have shown less benefit of chemotherapy with age.
Gene assays
The most recent advance in stage II colon cancer is the development of quantitative tumor gene expression to predict the risk of cancer recurrence in patients with stage II or III colon cancer (17). Oncotype DX Colon Cancer Assay and ColoPrint are gene expression profiling-based recurrence score (RS) assays that have been validated in a prospective study (18). The greatest utility of RS assay is in identifying recurrence risk in stage II patients.
Role of targeted therapies: bevacizumab has been tried in the adjuvant setting in stage II and III colon cancer in two prospective randomized trials (19,20) both showing no benefit of adjuvant chemotherapy. There is hence no role of targeted therapy in the adjuvant setting (despite success in the metastatic setting) which had been attributed to different molecular features of micrometastasis compared to macro metastatic disease (21).
Surveillance
The frequency of surveillance seems directly related to the early detection of colonic cancer. Delays in the detection of local recurrences directly affect resectability. Risk adapted follow-up is the best strategy. Recurrences were detected 8.5 months earlier with intensive follow-up and was associated with a reduction in all-cause mortality (22) and by itself confers a 5–7% survival benefit (level Ia evidence).
Conclusions
High risk stage II colon cancer does require adjuvant chemotherapy. Localized initial recurrences can be salvaged with combination of re-surgery and chemotherapy. A stringent surveillance is essential for picking up early recurrences and has an impact on survival which is re-emphasized by the index case.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Mamounas E, Wieand S, Wolmark N, et al. Comparative efficacy of adjuvant chemotherapy in patients with Dukes' B versus Dukes' C colon cancer: results from four National Surgical Adjuvant Breast and Bowel Project adjuvant studies (C-01, C-02, C-03, and C-04). J Clin Oncol 1999;17:1349-55. [PubMed]
- Wilkinson NW, Yothers G, Lopa S, et al. Long-term survival results of surgery alone versus surgery plus 5-fluorouracil and leucovorin for stage II and stage III colon cancer: pooled analysis of NSABP C-01 through C-05. A baseline from which to compare modern adjuvant trials. Ann Surg Oncol 2010;17:959-66. [Crossref] [PubMed]
- Weiss JM, Schumacher J, Allen GO, et al. Adjuvant chemotherapy for stage II right-sided and left-sided colon cancer: analysis of SEER-medicare data. Ann Surg Oncol 2014;21:1781-91. [Crossref] [PubMed]
- O'Connor ES, Greenblatt DY, LoConte NK, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol 2011;29:3381-8. [Crossref] [PubMed]
- Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J Clin Oncol 1999;17:1356-63. [PubMed]
- Tournigand C, André T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 2012;30:3353-60. [Crossref] [PubMed]
- Quasar Collaborative Group, Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020-9. [Crossref] [PubMed]
- Hermanek P. pTNM and residual tumor classifications: problems of assessment and prognostic significance. World J Surg 1995;19:184-90. [Crossref] [PubMed]
- Merkel S, Wein A, Günther K, et al. High-risk groups of patients with Stage II colon carcinoma. Cancer 2001;92:1435-43. [Crossref] [PubMed]
- Moertel CG, Fleming TR, Macdonald JS, et al. Intergroup study of fluorouracil plus levamisole as adjuvant therapy for stage II/Dukes' B2 colon cancer. J Clin Oncol 1995;13:2936-43. [PubMed]
- Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol 2003;21:2912-9. [Crossref] [PubMed]
- Wong JH, Severino R, Honnebier MB, et al. Number of nodes examined and staging accuracy in colorectal carcinoma. J Clin Oncol 1999;17:2896-900. [PubMed]
- Jen J, Kim H, Piantadosi S, et al. Allelic loss of chromosome 18q and prognosis in colorectal cancer. N Engl J Med 1994;331:213-21. [Crossref] [PubMed]
- Ng K, Schrag D. Microsatellite instability and adjuvant fluorouracil chemotherapy: a mismatch? J Clin Oncol 2010;28:3207-10. [Crossref] [PubMed]
- Donada M, Bonin S, Barbazza R, et al. Management of stage II colon cancer - the use of molecular biomarkers for adjuvant therapy decision. BMC Gastroenterol 2013;13:36. [Crossref] [PubMed]
- French AJ, Sargent DJ, Burgart LJ, et al. Prognostic significance of defective mismatch repair and BRAF V600E in patients with colon cancer. Clin Cancer Res 2008;14:3408-15. [Crossref] [PubMed]
- O'Connell MJ, Lavery I, Yothers G, et al. Relationship between tumor gene expression and recurrence in four independent studies of patients with stage II/III colon cancer treated with surgery alone or surgery plus adjuvant fluorouracil plus leucovorin. J Clin Oncol 2010;28:3937-44. [Crossref] [PubMed]
- Gray RG, Quirke P, Handley K, et al. Validation study of a quantitative multigene reverse transcriptase-polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J Clin Oncol 2011;29:4611-9. [Crossref] [PubMed]
- Allegra CJ, Yothers G, O'Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol 2011;29:11-6. [Crossref] [PubMed]
- de Gramont A, Van Cutsem E, Schmoll HJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol 2012;13:1225-33. [Crossref] [PubMed]
- Nelson VM, Benson AB 3rd. Status of targeted therapies in the adjuvant treatment of colon cancer. J Gastrointest Oncol 2013;4:245-52. [PubMed]
- Renehan AG, Egger M, Saunders MP, et al. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ 2002;324:813. [Crossref] [PubMed]
Cite this article as: Simha V, Kapoor R, Sharma S. Multiple localized metachronous recurrences in a patient of colon cancer and therapeutic controversies in stage II colon cancer. Transl Gastroenterol Hepatol 2016;1:20.


