Survival impact of the number of lymph node dissection on stage I–III node-negative gastric cancer
It is well known that lymph node (LN) status is the most important prognostic factor in localized gastric adenocarcinoma (GC) (1-4). Curative resection including adequate lymphadenectomy provided the chance of a cure for stage I–III disease (1,4,5). Unfortunately, a subgroup of patients with node-negative GC who underwent radical surgery including extensive LN dissection still experiences tumor recurrence, distant metastasis and subsequently died from the disease (6,7). In the issue of Annals of Surgery, Jin et al. indicated that in node-negative GC patients undergoing curative intent surgery, T3/T4 tumors, presence of lymphovascular invasion and signet ring histology independently affected overall survival suggesting that these patients may benefit from more aggressive adjuvant therapies (8). However, it should be noted that recurrence rates were 8.4% and 10.5% in T1 and stage I GC, respectively and 35.0% and 37.5% in T4 and stage III cancer, respectively in their study. The median number of nodes examined for patients with recurrence was 14 (range, 6–22), which might result in the possibility of underestimation of nodal involvement and understaging. In addition, tumor recurrence rates did not differ regardless of the extent of lymphadenectomy or the total nodes examined. The overall 5-year survival rate was 53% for the whole cohort.
Our previous study has shown that there was no survival benefit of >15 nodes retrieved for patients with T1 node-negative GC; however, patients with T2–4 node-negative GC with extensive lymphadenectomy (>25 nodal dissection) had longer survival time than those with nodes retrieved <25 (6). The GC-specific 5-year survival rates were 96.2%, 94.6%, and 97.9% in T1 tumor with the number of examined LN <15, 16–25 and >25, respectively (P=0.468). The overall 5-year survival rates were 74.0%, 81.9% and 84.4% for patients with T2, T3 and T4 node-negative GC, respectively. In contrast to the results of Jin et al., our large-scale study (n=1,030) indicated that tumor size, tumor location, the number of nodal retrieval, T4 status, and presence of perineural invasion were prognostic factors for T1–T4 node-negative GC based on multivariate analysis (7). The extent of lymphadenectomy and the number of LNs retrieved might explain the great survival discrepancy between Jin’s and our studies (7,8).
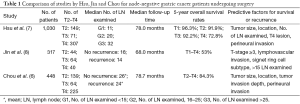
As T1 node-negative GC patients undergoing R0 resection had an excellent 5-year survival period and extremely low recurrence rates (7), we enrolled 448 T2–4 node-negative GC patients, who underwent radical resection (>10 nodes retrieved) without receiving neoadjuvant chemotherapy or postoperative irradiation therapy to identify determinants of tumor recurrence and to analyze the prognostic factors (6). Our results show that there was no significant difference in mean number of LN retrieved between GC patients without recurrence and with recurrence (26 vs. 24). The median follow-up time was 78.7 months. Recurrence was found in 85 patients (18.9%) in the whole cohort. Patients with T2, T3 and T4 tumor had recurrence rates of 8.6%, 12.5% and 26.5%. Tumor location, size, tumor invasion depth, and perineural invasion were associated with tumor recurrence and outcome. In contrast to our published article (6), Jin et al. included only 148 patients with T2–4 node-negative GC (n=148) and found that tumor recurrence rates were 9.1%, 29.7% and 35.0% in T2, T3 and T4 disease, respectively (8). The mean number of examined LN was 16 in patients with recurrence, which might result in inadequate lymphadenectomy in T2–T4 tumor and subsequently led to higher recurrence rates and worse survival time compared to our previous research (6). Furthermore, a limited sample size in Jin’s study might make the statistical difference insignificant between T2–T4 GC patients with less (< D2) and extensive (> D2) lymphadenectomy. Table 1 summarizes the key messages of node-negative GC studies by Hsu, Jin and Chou.
Full table
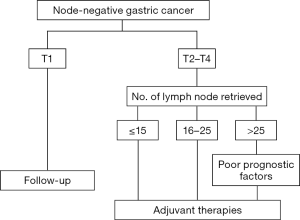
Figure 1 showed treatment strategies for stage I–III node-negative GC patients who underwent gastrectomy plus D1/D2 lymphadenectomy. For patients with poor prognostic factors for recurrence, such as a large tumor size, tumor involving the whole stomach, T4 lesion, presence of perineural invasion, and those with inadequate lymphadenectomy (<25 nodes retrieved for T2–T4 lesion), adjuvant therapies including chemotherapy and/or radiotherapy should be considered to improve patient outcome.
Acknowledgements
The authors thank Shu-Fang Huang for assisting in the statistical analysis and preparing the figure.
Funding: This work was partly supported by the Chang Gung Medical Research Program, Taiwan (CMRPG3C0602 and CORPG3E0151).
Footnote
Provenance: This is a Guest Commentary commissioned by the Section Editor Dr. Rulin Miao (Department of Gastrointestinal Surgery, Peking University Cancer Hospital & Institute, Beijing, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hsu JT, Chen TC, Tseng JH, et al. Impact of HER-2 overexpression/amplification on the prognosis of gastric cancer patients undergoing resection: a single-center study of 1,036 patients. Oncologist 2011;16:1706-13. [Crossref] [PubMed]
- Wang F, Chang YC, Chen TH, et al. Prognostic significance of splenectomy for patients with gastric adenocarcinoma undergoing total gastrectomy: a retrospective cohort study. Int J Surg 2014;12:557-65. [Crossref] [PubMed]
- Cheng CT, Tsai CY, Hsu JT, et al. Aggressive surgical approach for patients with T4 gastric carcinoma: promise or myth? Ann Surg Oncol 2011;18:1606-14. [Crossref] [PubMed]
- Hsu JT, Liu MS, Wang F, et al. Standard radical gastrectomy in octogenarians and nonagenarians with gastric cancer: are short-term surgical results and long-term survival substantial? J Gastrointest Surg 2012;16:728-37. [Crossref] [PubMed]
- Hsu JT, Liao CK, Le PH, et al. Prognostic Value of the Preoperative Neutrophil to Lymphocyte Ratio in Resectable Gastric Cancer. Medicine (Baltimore) 2015;94:e1589.
- Chou HH, Kuo CJ, Hsu JT, et al. Clinicopathologic study of node-negative advanced gastric cancer and analysis of factors predicting its recurrence and prognosis. Am J Surg 2013;205:623-30. [Crossref] [PubMed]
- Hsu JT, Lin CJ, Sung CM, et al. Prognostic significance of the number of examined lymph nodes in node-negative gastric adenocarcinoma. Eur J Surg Oncol 2013;39:1287-93. [Crossref] [PubMed]
- Jin LX, Moses LE, Squires MH 3rd, et al. Factors Associated With Recurrence and Survival in Lymph Node-negative Gastric Adenocarcinoma: A 7-Institution Study of the US Gastric Cancer Collaborative. Ann Surg 2015;262:999-1005. [Crossref] [PubMed]
Cite this article as: Hsu JT, Yeh TS, Jan YY. Survival impact of the number of lymph node dissection on stage I–III node-negative gastric cancer. Transl Gastroenterol Hepatol 2016;1:9.