Disseminated gastric carcinoma in disguise—presentation as microangiopathic haemolytic anemia with bone marrow necrosis
Introduction
Microangiopathic hemolytic anemia (MAHA) is a rare paraneoplastic syndrome in advanced adenocarcinomas. When a malignancy first presents as MAHA, it is often misdiagnosed and treated as thrombotic thrombocytopenic purpura (TTP). Progressive MAHA of unknown origin, therefore, warrants a search for occult malignancy. Bone marrow study in such cases may help locate the primary by unmasking tumor deposits, but extensive bone marrow necrosis (BMN) is another unusual finding in disseminated malignancy. However, both MAHA and BMN indicate grave prognosis in this setting.
Case presentation
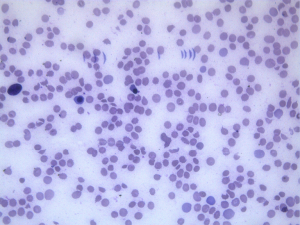
A 63-years-old male, chronic smoker, presented with non specific complaints of fatigue and decreased appetite of 6 months duration. On examination, he was pale. There were no other obvious findings. On routine blood examination, his haemoglobin level was only 7.9 g/dL with a mildly elevated WBC count of 12,200/mm3 and a low platelet count of 50,000/mm3. Peripheral smear revealed numerous schistocytes, spherocytes, polychromatophilic cells and normoblasts with thrombocytopenia suggestive of intravascular hemolysis (Figure 1).
Work-up for identifying the cause of haemolysis was initiated on an emergency basis. Liver function tests showed unconjugated hyperbilirubinemia with high levels of alkaline phosphatase (ALP)—2,638 U/L and lactate dehydrogenase (LDH)—1,241 U/L. Renal function tests were normal. Direct and indirect Coomb’s tests were negative ruling out immune mediated hemolysis. Coagulation profile as well as fibrinogen levels were also within normal limits thus excluding disseminated intravascular coagulation. The patient received supportive transfusions without significant improvement in blood counts. With TTP as a diagnostic consideration, plasma exchange was planned.
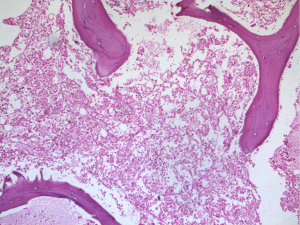
An urgent bone marrow study was done which showed diffuse coagulative necrosis of the marrow with near total absence of viable hematopoietic cells but preserved bony trabeculae (Figure 2). Meanwhile, the patient also underwent abdominal ultrasonography and subsequent gastrointestinal endoscopy which revealed suspicious pyloric thickening in the stomach. Hence plasma exchange was temporarily withheld and oncosurgery opinion sought.
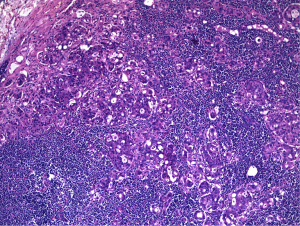
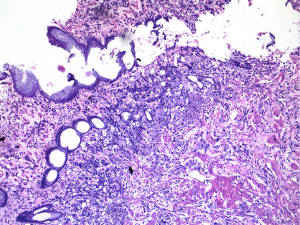
The oncosurgeon posted the patient for a diagnostic laparotomy wherein he proceeded with partial gastrectomy. The specimen showed an ulcer of 3.5 cm × 3 cm in the antral region with multiple enlarged lymph nodes in perigastric fat. Microscopy revealed a poorly differentiated adenocarcinoma with signet ring cells infiltrating almost upto the gastric serosa (Figure 3). Lymphovascular and perineural invasion were noted. 23 of 25 lymph nodes including the separately received cardiac and common hepatic nodes had tumor deposits with perinodal infiltrates (Figure 4).
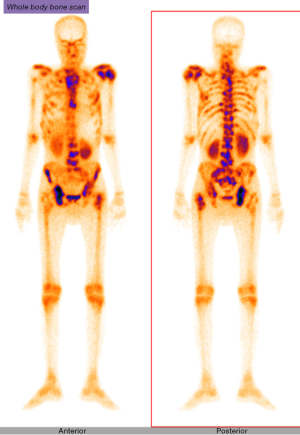
In the post operative period, he received multiple transfusions in view of the worsening counts but without improvement. The smear picture remained the same and the diagnosis of cancer related microangiopathic haemolytic anemia was established. Plasma exchange was not an option in this scenario. Prior to initiation of anti- cancer therapy, patient underwent a three phase bone scan to assess the extent of disease which reported disseminated skeletal metastases (Figure 5). Hence, the futility of any further cancer directed therapy was explained to carers and the patient discharged on palliative care on the 16th post operative day. Unfortunately, he succumbed after 2 days of discharge.
Discussion
Cancer-related microangiopathic hemolytic anemia (CR-MAHA) is a paraneoplastic syndrome characterized by Coombs-negative hemolytic anemia with schistocytes and thrombocytopenia. The exact incidence of this rare phenomenon is not known. In a review of 168 reported cases of CR-MAHA by Lechner and Obermeier in 2012, the more frequently associated malignancies were adenocarcinomas of stomach, breast, prostate and lung (1). Post operative and chemotherapy induced MAHA are not considered as true CR-MAHA.
The exact pathogenesis of CR-MAHA remains unclear. Red cell fragmentation and platelet destruction by microvascular tumor emboli, cytokine production by neoplastic cells, endothelial injury, impaired fibrinolysis are all implicated (2). The role of ADAMTS13 deficiency is limited and this explains the lack of response to plasma exchange therapy.
Where systemic malignancy is not initially apparent, the combination of microangiopathic hemolytic anemia and thrombocytopenia in CR-MAHA is often misdiagnosed as TTP, a medical emergency. The classical pentad of TTP includes fever, microangiopathic hemolytic anemia, thrombocytopenia, renal and neurological abnormalities. However, the urgency for its diagnosis in view of the effective early treatment options, has decreased the stringency of these criteria (3). This exposes patients to the risks of plasma exchange therapy which is effective for TTP, but inappropriate in CR-MAHA. In fact, disseminated malignancy is an alternative disorder that only mimics TTP and is not TTP associated (4).
In the Oklahoma TTP-HUS (TTP—hemolytic uremic syndrome) Registry, 10 (3%) of 351 patients managed preliminarily as TTP by exchange plasmapheresis were subsequently detected to have disseminated malignancy thereby ruling out TTP (5). Hence additional investigations like bone marrow examination, imaging studies and tumor markers are advisable in the evaluation of TTP.
In the review by Lechner and Obermeier, 81.1% of CR-MAHA cases with evaluable bone marrow studies showed varying degrees of tumor infiltration (1). However, bone marrow biopsy in our case revealed necrosis of hematopoietic tissue and stroma involving the entire core.
BMN is an ominous clinicopathological entity, rarely diagnosed antemortem. Though it has malignant and non malignant etiologies, the former predominate. According to Argon et al., malignancies constitute 91% of BMN cases wherein 60% are haematological malignancies and 31% solid tumors. The minority of non malignant causes include mainly severe infections, sickle cell disease, drugs, antiphospholipid syndrome and bone marrow transplant (6). Though the pathogenesis is controversial, several factors like microvascular infarction, granulocyte activation, excessive oxygen consumption by the rapidly proliferating tumor cells, tumor necrosis factor and thrombosis have all been suggested (7).
The overall prognosis and survival of patients with CR-MAHA and/or malignancy associated BMN are greatly inferior as both imply widespread disease. Early diagnosis and institution of chemotherapy would be the best treatment option for both, though with very limited benefit in most.
CR-MAHA and BMN are two rare adverse entities in disseminated malignancy. A review of literature on their pathogeneses points towards certain common culprits, but further studies are required to validate the same. Though recognised independently, their concurrent occurrence as in our case is extremely rare, and that too, as the presenting feature. This highlights the importance of awareness of these rare presentations and the need for tumor work up in such cases.
Acknowledgements
This work attributed to Malabar Institute of Medical Sciences Ltd., Calicut, Kerala.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Lechner K, Obermeier HL. Cancer-related microangiopathic hemolytic anemia: clinical and laboratory features in 168 reported cases. Medicine (Baltimore) 2012;91:195-205. [Crossref] [PubMed]
- Copelovitch L, Kaplan BS. The thrombotic microangiopathies. Pediatr Nephrol 2008;23:1761-7. [Crossref] [PubMed]
- George JN. How I treat patients with thrombotic thrombocytopenic purpura: 2010. Blood 2010;116:4060-9. [Crossref] [PubMed]
- Veyradier A, Meyer D. Thrombotic thrombocytopenic purpura and its diagnosis. J Thromb Haemost 2005;3:2420-7. [Crossref] [PubMed]
- Francis KK, Kalyanam N, Terrell DR, et al. Disseminated malignancy misdiagnosed as thrombotic thrombocytopenic purpura: A report of 10 patients and a systematic review of published cases. Oncologist 2007;12:11-9. [Crossref] [PubMed]
- Argon D, Cetiner M, Adiguzel C, et al. Bone marrow necrosis in a patient with non-. Hodgkin lymphoma. Turk J Haematol 2004;21:97-100.
- Elgamal BM, Rashed RA, Raslan HN. Prevalence of bone marrow necrosis in Egyptian cancer patients referring to the National Cancer Institute. J Egypt Natl Canc Inst 2011;23:95-9. [Crossref] [PubMed]
Cite this article as: Yesodharan J, Kuruvilla S, Parameswaran Kavitha K, Lilly M. Disseminated gastric carcinoma in disguise—presentation as microangiopathic haemolytic anemia with bone marrow necrosis. Transl Gastroenterol Hepatol 2016;1:6.